��Ŀ����
ʹһ����������������Ļ����������ͨ����֧���ȵ�Ӳ�ʲ����ܣ���һ֧��������װ��ͭм���ڶ�֧��������װ������ͭ����һ֧�����������ڷ������µķ�Ӧ��2PH3+3Cu![]() Cu3P2(��)+3H2�����������ʵ���������4.96g���ڶ�֧�����������ʵ�����������Ӧ�������5.76g��
Cu3P2(��)+3H2�����������ʵ���������4.96g���ڶ�֧�����������ʵ�����������Ӧ�������5.76g��
(1)����ԭ������������������������ȡ�
(2)�ڱ�״���£�ԭ���������ܶ��Ƕ��٣�
�𰸣�(1)4:3�� (2)0.91g/L
������
��ʾ��
������
����������ʵ�������x mol 2PH 2 mol�������������������� 62(2��34-6) x mol �������������������� 4.96 �ɵ÷���ʽ�� ͬ�������������y mol�� �������������������� 1mol�������������������� 16g �������������������� 0.24+y������������ �������� 5.76 �÷��̣�
|
��ʾ��
|

��ϰ��ϵ�д�
�����Ŀ
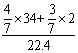 =0.92g/L��
=0.92g/L�� Cu3P2��s��+3H2�������������ʵ���������4.96g����2֧�����������ʵ�����������5.76g��
Cu3P2��s��+3H2�������������ʵ���������4.96g����2֧�����������ʵ�����������5.76g�� Cu3P2��s��+3H2�������������ʵ���������4.96g����2֧�����������ʵ�����������5.76g��
Cu3P2��s��+3H2�������������ʵ���������4.96g����2֧�����������ʵ�����������5.76g��