��Ŀ����
��Ҫ��������и��⣺
��1���õ���ʽ��ʾ���л�������γɹ���
H2S______��
MgF2______��
��2��������ͬ��H2O��D2O����������֮��Ϊ______��������֮��Ϊ______���ֱ��������Ľ����Ʒ�Ӧ����������ͬ��ͬѹ�����֮��Ϊ______��
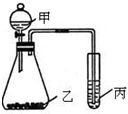
��3��������ͼװ�ò����ʵ��Լ������ij̽��ʵ�飬���ó���Ӧʵ����ۣ�����������Ϣ�ش�
��Ϊ��֤��Ԫ�صķǽ�����ǿ����S��C��Si������Ϊ������Ӧ���ǣ���Ϊ______����Ϊ______����Ϊ______��
�������Ϊˮ����ΪNa2O2��ĩ����ΪH2S�ı���ˮ��Һ��ʵ���й۲쵽�������ɵ���ɫ��������õ���ʵ������ǣ�______��
��1���õ���ʽ��ʾ���л�������γɹ���
H2S______��
MgF2______��
��2��������ͬ��H2O��D2O����������֮��Ϊ______��������֮��Ϊ______���ֱ��������Ľ����Ʒ�Ӧ����������ͬ��ͬѹ�����֮��Ϊ______��

��3��������ͼװ�ò����ʵ��Լ������ij̽��ʵ�飬���ó���Ӧʵ����ۣ�����������Ϣ�ش�
��Ϊ��֤��Ԫ�صķǽ�����ǿ����S��C��Si������Ϊ������Ӧ���ǣ���Ϊ______����Ϊ______����Ϊ______��
�������Ϊˮ����ΪNa2O2��ĩ����ΪH2S�ı���ˮ��Һ��ʵ���й۲쵽�������ɵ���ɫ��������õ���ʵ������ǣ�______��
��1����д���ӻ�����ĵ���ʽʱ������Ҫ������������������������Ҫ�ֿ�д�����ܺϲ���H2SΪ���ۻ�����γɹ���Ϊ
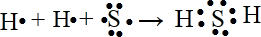
��MgF2Ϊ���ӻ�����õ���ʽ��ʾ���γɹ���Ϊ
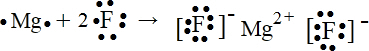
��
�ʴ�Ϊ��
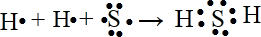
��
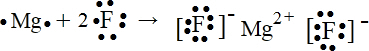
��
��2��������ͬ��H2O��D2O������ʵ���֮��Ϊ
��
=10��9������������֮��Ϊ10��9��������֮��Ϊ10��8��9��10=8��9���ɻ�ѧ����ʽ֪�������֮�ȵ���ˮ�����ʵ���֮�ȣ��ʴ�Ϊ��10��9�� 8��9��10��9
��3����Ԫ�طǽ�����ǿ������������Ӧ��ˮ��������Խǿ�����������̼����ᣬ����ǿ����ȡ�����ԭ��֪�ס��ҡ������ʴ�Ϊ�����ᡢ̼���ơ������ƣ���ˮ��������Ʒ�Ӧ�������������������Ա���ǿ������Ͷ��������Һ�пɽ����û��������ʴ�Ϊ�����ʵ�������ǿ��Ϊ��O2��S��
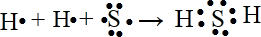
��MgF2Ϊ���ӻ�����õ���ʽ��ʾ���γɹ���Ϊ
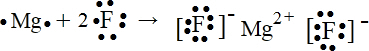
��
�ʴ�Ϊ��
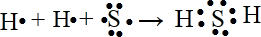
��
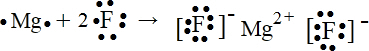
��
��2��������ͬ��H2O��D2O������ʵ���֮��Ϊ
| 1 |
| 18 |
| 1 |
| 20 |
��3����Ԫ�طǽ�����ǿ������������Ӧ��ˮ��������Խǿ�����������̼����ᣬ����ǿ����ȡ�����ԭ��֪�ס��ҡ������ʴ�Ϊ�����ᡢ̼���ơ������ƣ���ˮ��������Ʒ�Ӧ�������������������Ա���ǿ������Ͷ��������Һ�пɽ����û��������ʴ�Ϊ�����ʵ�������ǿ��Ϊ��O2��S��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ





 �ķ���ʽ��
�ķ���ʽ�� �л���Y��X��ͬ���칹�壬�����ڷ�������д��Y�Ľṹ��ʽ
�л���Y��X��ͬ���칹�壬�����ڷ�������д��Y�Ľṹ��ʽ