��Ŀ����
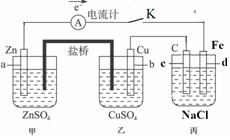
 ��ͼװ�ñպϵ��Kʱ��������A��ָ�뽫����ƫת���Իش�
��ͼװ�ñպϵ��Kʱ��������A��ָ�뽫����ƫת���Իش���1��������
��2������Cu���ĵ缫��Ӧ��
��3���պϵ��Kһ��ʱ����������ɶ��������һ�ּ������з�
�����ܵĻ�ѧ����ʽ��
��4�������з�Ӧ���нϳ�ʱ����ռ�����״��������2.24L��ʱ��ñ�����Һ����ʵ�ʼ���4.23g�����м�0.100mol��������������ˮ�е��ܽ⣩����ʵ�ʷų���������ʵ�����
��5�����Ҫ��������Ƭ�϶���һ��Cu�������Ӧ���θĽ�
��������1�������γ�ԭ��أ����Ա�����ӵ�Դ�����ڵ��أ�����aʧ����Ϊ����������c�缫����ԭ��ص���������������
��2������Cu���������ӵõ��ӣ����ݵ�����Zn�����ʵ�����ϵ���㣻
��3���������ǵ��NaCl��Һ�����������������������ƣ�
��4�����ݵ��NaCl��Һ�����������������������ƵĹ�ϵ����֪��0.100mol�������������ɱ�����Һ����ʵ�ʼ��ٵ������������������
��5��Ҫ��������Ƭ�϶���һ��Cu��������Ӧ��ΪCu�缫���������ҺΪ����ͭ��Һ��
��2������Cu���������ӵõ��ӣ����ݵ�����Zn�����ʵ�����ϵ���㣻
��3���������ǵ��NaCl��Һ�����������������������ƣ�
��4�����ݵ��NaCl��Һ�����������������������ƵĹ�ϵ����֪��0.100mol�������������ɱ�����Һ����ʵ�ʼ��ٵ������������������
��5��Ҫ��������Ƭ�϶���һ��Cu��������Ӧ��ΪCu�缫���������ҺΪ����ͭ��Һ��
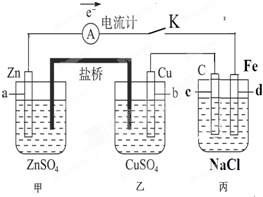
����⣺��1�������γ�ԭ��أ����Ա�����ӵ�Դ�����ڵ��أ�����aʧ����Ϊ����������c�缫����ԭ��ص����������������ʴ�Ϊ�����أ�������������
��2������Cu����ͭ���ӵõ�������ͭ���ʣ���缫��ӦΪ��Cu2++2e-=Cu��Zn��0�����ߵ�+2��ʧȥ2�����ӣ���֪��·����0.02mol����ͨ�����������ĵ�ZnΪ0.01mol��m��Zn��=nM=65g/mol��0.01mol=0.65g���ʴ�Ϊ��0.65g��
��3���������ǵ��NaCl��Һ�����������������������ƣ����ⷽ��ʽ��2NaCl+2H2O
2NaOH+H2��+Cl2����
�ʴ�Ϊ��2NaCl+2H2O
2NaOH+H2��+Cl2����
��4����֪��0.100mol��n��H2��=
=
=0.1mol
2NaCl+2H2O
2NaOH+H2��+Cl2����
0.1mol 0.05mol
����m��H2��=nM=0.1mol��2g/mol=0.2g��m�� Cl2��=nM=0.05mol��71g/mol=3.55g��������Һ����ʵ�ʼ���4.23g���������������������������ͣ�
����m��O2��=4.23g-3.55g-0.2g=0.48g������n��O2��=
=
=0.015mol��
���ʵ�ʷų���������ʵ����ǣ�0.1mol+0.05mol+0.015mol=0.165mol���ʴ�Ϊ��0.165��
��5��Ҫ��������Ƭ�϶���һ��Cu��������Ӧ��ΪCu�缫���������ҺΪ����ͭ��Һ���ʴ�Ϊ����C�缫����Cu�缫����NaCl��Һ��Ϊ����ͭ��Һ��
��2������Cu����ͭ���ӵõ�������ͭ���ʣ���缫��ӦΪ��Cu2++2e-=Cu��Zn��0�����ߵ�+2��ʧȥ2�����ӣ���֪��·����0.02mol����ͨ�����������ĵ�ZnΪ0.01mol��m��Zn��=nM=65g/mol��0.01mol=0.65g���ʴ�Ϊ��0.65g��
��3���������ǵ��NaCl��Һ�����������������������ƣ����ⷽ��ʽ��2NaCl+2H2O
| ||
�ʴ�Ϊ��2NaCl+2H2O
| ||
��4����֪��0.100mol��n��H2��=
| V |
| Vm |
| 2.24L |
| 22.4L/mol |
2NaCl+2H2O
| ||
0.1mol 0.05mol
����m��H2��=nM=0.1mol��2g/mol=0.2g��m�� Cl2��=nM=0.05mol��71g/mol=3.55g��������Һ����ʵ�ʼ���4.23g���������������������������ͣ�
����m��O2��=4.23g-3.55g-0.2g=0.48g������n��O2��=
| m |
| M |
| 0.48g |
| 32g/mol |
���ʵ�ʷų���������ʵ����ǣ�0.1mol+0.05mol+0.015mol=0.165mol���ʴ�Ϊ��0.165��
��5��Ҫ��������Ƭ�϶���һ��Cu��������Ӧ��ΪCu�缫���������ҺΪ����ͭ��Һ���ʴ�Ϊ����C�缫����Cu�缫����NaCl��Һ��Ϊ����ͭ��Һ��
�����������ۺϿ���ԭ��غ͵���֪ʶ���Ǹ߿��еij������ͺ���Ҫ�Ŀ���֮һ�������е��Ѷȵ����⣮�����ۺ���ǿ���������У������߿�������������ѧ���������⡢��������������Ҳ����������ѧ������˼ά�����ͷ�ɢ˼ά���������ѧ��ѧϰЧ�ʣ�

��ϰ��ϵ�д�
�����Ŀ