��Ŀ����
��֪���������������·�Ӧ��ϵ��(1)KBrO3�ܽ�KI������I2��KIO3���䱾������ԭΪBr2��
(2) Br2�ܽ�I-����ΪI2��
(3)KIO3�ܽ�I-����ΪI2��Ҳ�ܽ�Br-����ΪBr2���䱾������ԭΪI2��
��������(Br2��KBrO3��KlO3)����������ǿ������˳��Ϊ��____________________��
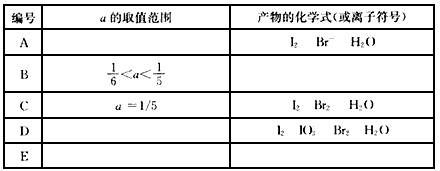
��������1molKI��������Һ�м��뺬amolKBrO3��Һ��a��ȡֵ��ͬ�����ò���Ҳ��ͬ���Խ����۵Ľ�������±��У�
��� | a��ȡֵ��Χ | ����Ļ�ѧʽ(�����ӷ���) |
A |
| I2 Br- H2O |
B |
|
|
C | a=1/5 | I2 Br2 H2O |
D |
| I2 |
E |
|
|
���������еⵥ�ʺ��嵥�ʵ����ʵ������ʱ��a��ֵΪ��_____________��
��KBrO3��KIO3��Br2
��A.a��![]() B. I2��Br-��Br2��H2O
B. I2��Br-��Br2��H2O
D.![]() ��a��
��a��![]() E.a��
E.a��![]()
![]() ��Br2��H2O
��Br2��H2O
��![]()
���������⿼���漰������ϵ��������ԭ����ʽ����д
![]() +6I-+6H+====3I2+Br-+3H2O
+6I-+6H+====3I2+Br-+3H2O
2![]() +10I-+12H+====5I2+Br2+6H2O
+10I-+12H+====5I2+Br2+6H2O
6![]() +5I-+6H+====5
+5I-+6H+====5![]() +3Br2+3H2O
+3Br2+3H2O
����������������ͬ�����ɵIJ���Ҳ��ͬ

��ϰ��ϵ�д�
 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д� ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д�
�����Ŀ