��Ŀ����
��1mol I2��g����2mol H2��g������ij2L�ܱ������У���һ���¶��·�����Ӧ��
I2��g��+H2��g��![]() 2HI��g��; ��H<0�����ﵽƽ�⡣HI�������������HI����ʱ��仯��ͼ���ߣ�II����ʾ��
2HI��g��; ��H<0�����ﵽƽ�⡣HI�������������HI����ʱ��仯��ͼ���ߣ�II����ʾ��

��1����ƽ��ʱ��I2��g�������ʵ���Ũ��Ϊ mol?L-1��
��2�����ı䷴Ӧ��������ij�����¦���HI���ı仯������
��I����ʾ��������������� ������������������ţ���
�ٺ��������£������¶�
�ں��������£������¶�
�ۺ��������£���С��Ӧ�������
�ܺ��������£�����Ӧ�������
�ݺ��¡����������£������ʵ�����
��3���������¶Ȳ��䣬����һ��ͬ��2L�ܱ������м���a mol I2��g����b mol H2��g����c mol HI��g����a��b��c������0����������Ӧ��ƽ��ʱ��HI�����������Ϊ0.60,��a��b��c�Ĺ�ϵΪ ����һ����a��b��c�Ĵ���ʽ��ʾ����
��4������ʱ��0.01 mol HI��������ˮ���100 ml��Һ�������Һ����ˮ��������������ӵ����ʵ���Ũ��Ϊ mol?L-1 ���������¶ȸ���Һ��pHֵ�� ��������С�䣩
��1�� 0.05��
��2���ۢݣ�
��3��2b-4a=c
��4��1��10��13 ����

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д� ��1mol I2��g�� ��2mol H2����2L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g������H��0������ƽ�⣮HI���������w��HI����ʱ��仯��ͼ���ߣ�����ʾ��
��1mol I2��g�� ��2mol H2����2L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g������H��0������ƽ�⣮HI���������w��HI����ʱ��仯��ͼ���ߣ�����ʾ�� ��1mol I2��g����2mol H2����5L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g����H��0�����ﵽƽ�⣮HI���������w��HI����ʱ��仯�����ߣ�����ʾ��
��1mol I2��g����2mol H2����5L�ܱ������У���һ���¶��·�����Ӧ��I2��g��+H2��g��?2HI��g����H��0�����ﵽƽ�⣮HI���������w��HI����ʱ��仯�����ߣ�����ʾ��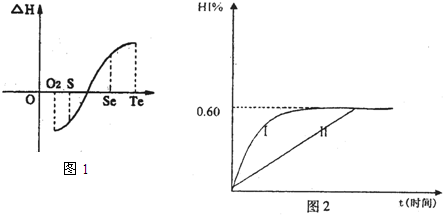

 ��
�� ��2H2O
��2H2O Al��OH��3�� ��NH3��H2O�����е����ʵ�����
Al��OH��3�� ��NH3��H2O�����е����ʵ����� 2HI��g������H<0�� ����ƽ�⡣HI���������HI����ʱ�ʱ仯������ͼ2��ʾ��
2HI��g������H<0�� ����ƽ�⡣HI���������HI����ʱ�ʱ仯������ͼ2��ʾ��