��Ŀ����
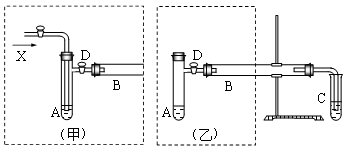
��12�֣�ʵ���ҿ������Ҵ���ͭ��ͭ�Ļ������Ʊ���ȩ����ͼ��ij��ȤС����Ƶ�ʵ��װ�ã��Ҳ�ķ�Ӧװ����ͬ�����������巢��װ�ò�ͬ���Թ�C��װ��ˮ������װ��δ���������Իش�
(1) ����װ���е�A��B��������ȣ�A����ˮԡ���ȣ�B����__________���ȣ�A����ˮԡ���ȵ���Ҫ�ŵ���__________��
(2) ������װ�ý���ʵ�飬B�ܴ�װͭ�ۣ���ͨ��A�ܵ�X��__________��B�з�Ӧ�Ļ�ѧ����ʽ��__________��
(3) ������װ�ý���ʵ�飬��B����Ӧװ__________��B�з�Ӧ�Ļ�ѧ����ʽ��__________��
(4) ʵ�������ȡC�Թ��е���Һ�������Ʊ���������Һ������ˮԡ���ȿɹ۲쵽���������ɣ�д���ù��̵����ӷ���ʽ__________ ��
(5) ʵ����ɺ�Ӧ��C�Թ��м���__________��ϴȥ������
(1)�ƾ��ƻ�ú���ơ��������Ⱦ��ȣ������ȶ���
(2)O2�������������CH3CH2OH+O2 2CH3CHO+2H2O
2CH3CHO+2H2O
(3)CuO CH3CH2OH+CuO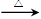 CH3CHO+Cu+H2O
CH3CHO+Cu+H2O
(4)CH3CHO+2Ag(NH3)2+
+ 2OH- 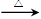 CH3COO- +NH4+
+2Ag+3NH3+H2O
CH3COO- +NH4+
+2Ag+3NH3+H2O
(5)HNO3
��������ˮԡ�����Ҵ������Ⱦ��ȣ��Ҵ�������Ҫ�ƾ��Ƽ��ȡ�������ӦҪ�ȼӼ�
CuOCH3CH2OH+CuO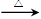 CH3CHO+Cu+H2O
CH3CHO+Cu+H2O
(4)CH3CHO+2Ag(NH3)2+
+ 2OH-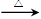 CH3COO- +NH4+
+2Ag+3NH3+H2O
CH3COO- +NH4+
+2Ag+3NH3+H2O
ʵ����ɺ�Ӧ��C�Թ��м���ϡ�����ϴȥ������

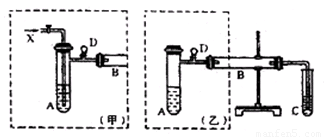
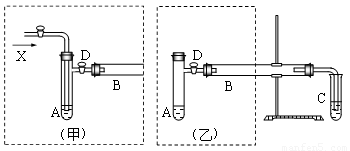
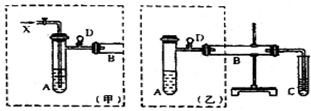 ʵ���ҿ������Ҵ���ͭ��ͭ�Ļ������Ʊ���ȩ����ͼ��ij��ȤС����Ƶ�ʵ��װ�ã��ұߵķ�Ӧװ����ͬ������ߵ����巢��װ�ò�ͬ���Թ�C��װ��ˮ������װ��δ���������Իش�
ʵ���ҿ������Ҵ���ͭ��ͭ�Ļ������Ʊ���ȩ����ͼ��ij��ȤС����Ƶ�ʵ��װ�ã��ұߵķ�Ӧװ����ͬ������ߵ����巢��װ�ò�ͬ���Թ�C��װ��ˮ������װ��δ���������Իش�