��Ŀ����
ij����С��Ϊ̽��CO2�������NaOH��Һȷʵ�����˻�ѧ��Ӧ���ס��ҡ�����λͬѧ�������������ʵ��װ�ã���ش��������⣺
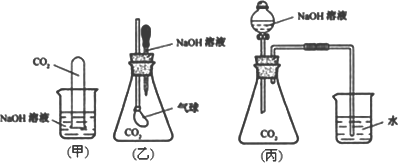
(1)��װ����CO2�������NaOH��Ӧ�����ӷ���ʽ________��
(2)��װ��ʵ��������________�����Ͳ�����ʵ�������ԭ��________��
(3)�ס��ҡ���ͬѧ��Ƶ����������У���һ��������ʵ�ʲ����а�ȫ�Դ������⣬�÷�����________(��ס��һ��)��ԭ����________��
(4)�����һ��ʵ��������ɵIJ���Na2CO3�е������ӣ�(�����������衢�����Լ���ʵ������ͽ��ۣ�)
(5)ʵ������������44.8 L(��״̬)CO2�����ú�CaCO3��90��ʯ��ʯ�����������ᷴӦ��������Ҫ����ʯ��ʯ________g(�������һλС��)��
������
|
����(1)CO2��2OH�� ����(2)��NaOH��Һ������ƿ֮���ձ��е�ˮ��������ƿ(1��)��NaOH��CO2��Ӧ��������ƿ��ѹǿ���ͣ��ڴ���ѹ�������£��ձ��е�ˮ��������ƿ(1��) ����(3)��(1��)��NaOH��CO2��Ӧ����ƿ��ѹǿ���ͣ��ڴ���ѹ�������£������ʹ��ܻᷢ����ը(1��) ����(4)ȡ�������������Һ���Թܣ��������ᣬ������ɫ��ζ�����壬����Һ�к���CO32��(2��) ����(5)222.2(2��) |





