��Ŀ����
����Ŀ���Լ״�Ϊȼ�ϵ����͵�أ���ɱ�������������Ϊȼ�ϵĴ�ͳȼ�ϵ�أ�Ŀǰ�õ��㷺���о�����ͼ��Ŀǰ�о��϶��һ�����������ȼ�ϵ�ع���ԭ��ʾ��ͼ���ش��������⣺

��1��B���ϵĵ缫��ӦʽΪ___________________________��
��2�����ø�ȼ�ϵ������Դ����ʯī���缫�������ͭ��Һ������ʱ�����ķ�ӦʽΪ_______________________________________���������ռ���11.2L����״��������ʱ�����ļ״�������Ϊ__________��(������λ��Ч����)����Ҫʹ��Һ��ԭ������������Һ�м���������� ___________��
��3��Ŀǰ�ѿ������õ�ⷨ��ȡClO2���¹��ա�
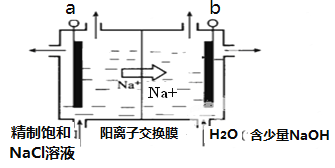
����ͼʾ����ʯī���缫����һ�������µ�ⱥ��ʳ��ˮ��ȡClO2�����������״�ȼ�ϵ�ؽ��е�⣬����صĵ缫a�Ӽ״�ȼ�ϵ�ص�________������A��B����д����������ClO2�ĵ缫��Ӧʽ��__________��
�ڵ��һ��ʱ�䣬�������������������Ϊ112 mL����״����ʱ��ֹͣ��⡣ͨ�������ӽ���Ĥ�������ӵ����ʵ���Ϊ_________mol��
���𰸡�3O2-+CH3OH-6e-=CO2+2H2O 2H2O-4e-=4H++O210.67CuO��CuCO3A 4OH-+Cl--5e- =ClO2+2H2O0.01
��������
��1�����������ƶ������֪BΪ��������������������Ӧ���״����������ɶ�����̼��ˮ���缫����ʽΪ3O2-+CH3OH-6e-=CO2+2H2O���ʴ�Ϊ��3O2-+CH3OH-6e-=CO2+2H2O����2���ö��Ե������ͭ��Һʱ������Ϊˮ�����OH-�ŵ磬�缫��ӦʽΪ��2H2O-4e-=4H++O2������������11.2L����ʱ��ת�Ƶĵ���Ϊ11.2L��22.4L/mol��4=2mol���ɵ����غ�ɵü״�������Ϊ2mol��6��32g/mol=10.67g���ڵ������ͭ��Һ�Ĺ����У���������ͭ������������������Ҫʹ��Һ��ԭ������ԭ���غ��֪Ӧ����Һ�м���CuO��CuCO3���ʴ�Ϊ��2H2O-4e-=4H++O2��10.67��CuO��CuCO3����3���ٸ��������ӵ��ƶ������֪a��Ϊ���ص����������Դ�����������ӣ��ʽ�A����������Ϊ�����ӷŵ磬��������ΪClO2����缫��ӦΪ��4OH-+Cl--5e- =ClO2+2H2O���ʴ�Ϊ��A��4OH-+Cl--5e- =ClO2+2H2O����H���������Ϸŵ����������ת�Ƶ��ӵ����ʵ���n(e��)��2n(H2)��2��(0.112 L��22.4 L��mol��1)��0.01 mol�������ڵ�·���ƶ��ĵ��Ϊ0.01 mol��ÿ��Na����һ����λ������ɣ���ͨ����Na��Ϊ0.01 mol���ʴ�Ϊ��0.01��