��Ŀ����
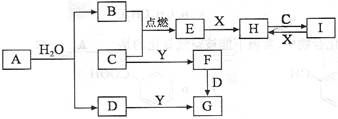
ij�������ǽ�������A�������ַǽ���Ԫ����ɣ���������Ԫ�صĻ��ϼ�Ϊ��������ۻ�����ۣ�����һ�ֳ�Ӳ���ʣ�������ĥ����ʴ�������ȳ������������������A���ɻ�����B�����з�Ӧ�Ƶã���B��NH3��C[C�Ļ�ѧʽΪSi(NH2)4]����C�������������·ֽ�õ�A��Ϊ̽��B����ɣ���������ͼ��ʾ��ת��ʵ�飬ͼ��G��F��H��Ϊ������ˮ�����ʣ���Ϊ��ɫ��ĩ��ͼ����ĸ�����ľ�Ϊ��ѧ��ѧ���������ʡ�

��ش��������⣺
(1)д��������B�ͻ�����G�Ļ�ѧʽ________��________��
(2)A���������ľ���������________����A�����У�ÿ��������ԭ����Χ��ϵ���һ��ԭ�ӵĸ�����________��
(3)д����Ӧ�ڵ����ӷ���ʽ��_____________________________________________��
(4)д��C�������������·ֽ�õ�A�Ļ�ѧ����ʽ��_________________________________________________��
(5)������Ӧ�ۣ����ܵó��Ľ�����________(��������)��

��ش��������⣺
(1)д��������B�ͻ�����G�Ļ�ѧʽ________��________��
(2)A���������ľ���������________����A�����У�ÿ��������ԭ����Χ��ϵ���һ��ԭ�ӵĸ�����________��
(3)д����Ӧ�ڵ����ӷ���ʽ��_____________________________________________��
(4)д��C�������������·ֽ�õ�A�Ļ�ѧ����ʽ��_________________________________________________��
(5)������Ӧ�ۣ����ܵó��Ľ�����________(��������)��
(1)SiCl4��AgCl
(2)ԭ�Ӿ��塡4
(3)SiO2��2OH��=SiO32-��H2O
(4)3Si(NH2)4 Si3N4��8NH3��
Si3N4��8NH3��
(5)H2CO3�����Ա�H2SiO3ǿ(��C�ķǽ����Աȹ�ǿ��)
(2)ԭ�Ӿ��塡4
(3)SiO2��2OH��=SiO32-��H2O
(4)3Si(NH2)4
 Si3N4��8NH3��
Si3N4��8NH3��(5)H2CO3�����Ա�H2SiO3ǿ(��C�ķǽ����Աȹ�ǿ��)
�� ��֪��E�к���Cl������BΪ�Ȼ����
��֪��E�к���Cl������BΪ�Ȼ���� ��֪��F��һ�����Ա�̼�����ķǽ����������Ӧ��ˮ�����Ϊ�����ԭ���ᣬ��CΪ�軯����BΪSiCl4��Si(NH2)4�����������·ֽ�ɵ�A����֪A����Si��N��ɵĻ�������ݹ�͵�Ԫ�صļ�̬��֪A�Ļ�ѧʽΪSi3N4����һ��������ΪNH3���ݴ˿ɵ�C�ֽ�Ļ�ѧ����ʽΪ��3Si(NH2)4
��֪��F��һ�����Ա�̼�����ķǽ����������Ӧ��ˮ�����Ϊ�����ԭ���ᣬ��CΪ�軯����BΪSiCl4��Si(NH2)4�����������·ֽ�ɵ�A����֪A����Si��N��ɵĻ�������ݹ�͵�Ԫ�صļ�̬��֪A�Ļ�ѧʽΪSi3N4����һ��������ΪNH3���ݴ˿ɵ�C�ֽ�Ļ�ѧ����ʽΪ��3Si(NH2)4 Si3N4��8NH3����
Si3N4��8NH3����
 ��֪��E�к���Cl������BΪ�Ȼ����
��֪��E�к���Cl������BΪ�Ȼ���� ��֪��F��һ�����Ա�̼�����ķǽ����������Ӧ��ˮ�����Ϊ�����ԭ���ᣬ��CΪ�軯����BΪSiCl4��Si(NH2)4�����������·ֽ�ɵ�A����֪A����Si��N��ɵĻ�������ݹ�͵�Ԫ�صļ�̬��֪A�Ļ�ѧʽΪSi3N4����һ��������ΪNH3���ݴ˿ɵ�C�ֽ�Ļ�ѧ����ʽΪ��3Si(NH2)4
��֪��F��һ�����Ա�̼�����ķǽ����������Ӧ��ˮ�����Ϊ�����ԭ���ᣬ��CΪ�軯����BΪSiCl4��Si(NH2)4�����������·ֽ�ɵ�A����֪A����Si��N��ɵĻ�������ݹ�͵�Ԫ�صļ�̬��֪A�Ļ�ѧʽΪSi3N4����һ��������ΪNH3���ݴ˿ɵ�C�ֽ�Ļ�ѧ����ʽΪ��3Si(NH2)4 Si3N4��8NH3����
Si3N4��8NH3����
��ϰ��ϵ�д�
�����Ŀ
 P
P O
O N
N C
C