��Ŀ����
(12��)����ѧ�������ʽṹ�����ʡ�
ͭ���ʼ��仯�����ںܶ���������Ҫ��;�������ͭ����������ߵ��£���ˮ������ͭ������ɱ�����ȡ�
��1��Cu2+�ĺ�������Ų�ʽΪ________________��
��2����ѧ��ͨ��X���߲���мȺ�����λ�����ֺ����������ṹʾ��ͼ�ɼ�ʾ���£�

�ٵ����Ļ�ѧʽ����������ʽ��ʾΪ______��
�ڵ�����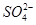 �Ŀռ乹��Ϊ_________��H2O��Oԭ�ӵ��ӻ���ʽΪ____________��
�Ŀռ乹��Ϊ_________��H2O��Oԭ�ӵ��ӻ���ʽΪ____________��
��3��������ͭ��Һ�м��������ˮ�������� �����ӡ���֪
�����ӡ���֪ �Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ����____________________________��
�Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ����____________________________��
��4�� N�γɵľ���ṹ��ͼ��ʾ��N3-����λ����________���辧���߳�Ϊa cm���ܶ�Ϊb g/cm3�����ӵ������ɱ�ʾΪ___________(�ú�a��b��ʽ�ӱ�ʾ)��
N�γɵľ���ṹ��ͼ��ʾ��N3-����λ����________���辧���߳�Ϊa cm���ܶ�Ϊb g/cm3�����ӵ������ɱ�ʾΪ___________(�ú�a��b��ʽ�ӱ�ʾ)��

ͭ���ʼ��仯�����ںܶ���������Ҫ��;�������ͭ����������ߵ��£���ˮ������ͭ������ɱ�����ȡ�
��1��Cu2+�ĺ�������Ų�ʽΪ________________��
��2����ѧ��ͨ��X���߲���мȺ�����λ�����ֺ����������ṹʾ��ͼ�ɼ�ʾ���£�

�ٵ����Ļ�ѧʽ����������ʽ��ʾΪ______��
�ڵ�����
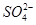 �Ŀռ乹��Ϊ_________��H2O��Oԭ�ӵ��ӻ���ʽΪ____________��
�Ŀռ乹��Ϊ_________��H2O��Oԭ�ӵ��ӻ���ʽΪ____________����3��������ͭ��Һ�м��������ˮ��������
 �����ӡ���֪
�����ӡ���֪ �Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ����____________________________��
�Ŀռ乹�Ͷ��������Σ���NF3������Cu2+�γ������ӣ���ԭ����____________________________����4��
 N�γɵľ���ṹ��ͼ��ʾ��N3-����λ����________���辧���߳�Ϊa cm���ܶ�Ϊb g/cm3�����ӵ������ɱ�ʾΪ___________(�ú�a��b��ʽ�ӱ�ʾ)��
N�γɵľ���ṹ��ͼ��ʾ��N3-����λ����________���辧���߳�Ϊa cm���ܶ�Ϊb g/cm3�����ӵ������ɱ�ʾΪ___________(�ú�a��b��ʽ�ӱ�ʾ)��
��1��[Ar]3d9
��2����[Cu(H2O)4]SO4��H2O ������������ sp3
��3��F�ĵ縺�Ա�N��N-F�ɼ����Ӷ�ƫ��Fԭ�ӣ�ʹ�õ�ԭ���ϵŶԵ�������Cu2+�γ�������
��4��6 206/a3b
��2����[Cu(H2O)4]SO4��H2O ������������ sp3
��3��F�ĵ縺�Ա�N��N-F�ɼ����Ӷ�ƫ��Fԭ�ӣ�ʹ�õ�ԭ���ϵŶԵ�������Cu2+�γ�������
��4��6 206/a3b
�����������1��ͭΪ29��Ԫ�أ����̬ԭ�Ӻ�������Ų�Ϊ[Ar]3d104s1������3d��4s����ֱ�Ϊȫ�����Ͱ��������Cu2+�ĺ�������Ų�Ϊ[Ar]3d9����2�����ݵ����Ľṹʾ��ͼ��֪Cu2+��4��H2O�е���ԭ���γ���λ���������е�2��ˮ������������һ��H2O�������ϣ�Ȼ������������������ϣ��ʿɱ�ʾΪ��[Cu(H2O)4]SO4��H2O������ͼʾ��֪������
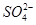 �Ŀռ乹��ӦΪ���������ͣ�H2O��Oԭ�ӵ��ӻ���ʽΪsp3����3����ΪF�ĵ縺�Ա�N��N-F�ɼ����Ӷ�ƫ��Fԭ�ӣ�ʹ�õ�ԭ���ϵŶԵ�������Cu2+�γ������ӣ���4�����ݾ����ṹ��֪����λ��ΪN3-������ΪCu+����N3-����λ����6��1�������к���3��Cu+��1��N3������1������������Ϊ206/NA g�����Ϊa3 cm3�������ܶ�bg/cm3=206/NA g��a3 cm3����NA=206/a3b��
�Ŀռ乹��ӦΪ���������ͣ�H2O��Oԭ�ӵ��ӻ���ʽΪsp3����3����ΪF�ĵ縺�Ա�N��N-F�ɼ����Ӷ�ƫ��Fԭ�ӣ�ʹ�õ�ԭ���ϵŶԵ�������Cu2+�γ������ӣ���4�����ݾ����ṹ��֪����λ��ΪN3-������ΪCu+����N3-����λ����6��1�������к���3��Cu+��1��N3������1������������Ϊ206/NA g�����Ϊa3 cm3�������ܶ�bg/cm3=206/NA g��a3 cm3����NA=206/a3b��
��ϰ��ϵ�д�
 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�
�����Ŀ