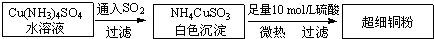��Ŀ����
CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȣ���֪��
��CuCl������CuCl2���ʵ��Ļ�ԭ����SO2��SnCl2�Ȼ�ԭ�Ƶã�
2Cu2+��2Cl����SO2��2H2O![]() 2CuCl����4H����SO
2CuCl����4H����SO![]()
2CuCl2��SnCl2��2CuCl����SnCl4
��CuCl2��Һ���Ҷ���(H2N��CH2��CH2��NH2)���γ������ӣ�

��ش��������⣺
(1)��̬Cuԭ�ӵĺ�������Ų�ʽΪ________��H��N��O����Ԫ�صĵ縺���ɴ�С��˳����________��
(2)SO2���ӵĿռ乹��Ϊ________����SnCl4��Ϊ�ȵ������һ�����ӵĻ�ѧʽΪ________��
(3)�Ҷ��������е�ԭ�ӹ�����ӻ�����Ϊ________���Ҷ��������װ�[N(CH3)3]�����ڰ������Ҷ��������װ��ķе�ߵĶ࣬ԭ����________��
(4)�������γɵ��������к��еĻ�ѧ��������________��(����ĸ)
a�����
b�����Լ�
c�����Ӽ�
d���Ǽ��Լ�
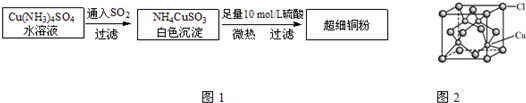
(5)CuCl�ľ����ṹ��ͼ��ʾ������Clԭ�ӵ���λ��Ϊ________��

�𰸣�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ



 ͭ���ʼ��仯�����ںܶ���������Ҫ����;�������ͭ����������ߵ��£���ϸͭ�ۿ�Ӧ���ڵ�����ϡ������������У�CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȣ�
ͭ���ʼ��仯�����ںܶ���������Ҫ����;�������ͭ����������ߵ��£���ϸͭ�ۿ�Ӧ���ڵ�����ϡ������������У�CuCl��CuCl2������Ҫ�Ļ���ԭ�ϣ����������������ϡ����������������ȣ�