��Ŀ����
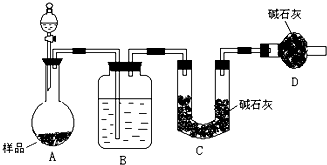
ijУ��ѧ�о�ѧϰС���ͬѧ��ʵ������Ũ�����MnO2��ȡ��̽��Cl2����ˮ�IJ������ʣ������������������⣺ʵ��һ����������ȡ�����ʣ�ʵ��װ������ͼ��

��1��A����������װ�ã����˾ƾ�����õ�����������_________________��
��2��ͬѧ����ʵ���з��֣�����ϡ�������Ũ������MnO2��ϼ���û���������ɡ���Ӱ���������ɵ�ԭ����ʲô�أ���ͬѧ����������̽����
������������
����1��Cl����Ũ�ȿ��ܶԷ�Ӧ��Ӱ��
����2��________________
�����ʵ�鷽��������ʵ�顣
������±���ʵ�鲽���Լ�Ԥ������ͽ��ۡ�
��ѡʵ���Լ��� ŨH2SO4��NaCl���塢MnO2���塢ϡ����
��2��ͬѧ����ʵ���з��֣�����ϡ�������Ũ������MnO2��ϼ���û���������ɡ���Ӱ���������ɵ�ԭ����ʲô�أ���ͬѧ����������̽����
������������
����1��Cl����Ũ�ȿ��ܶԷ�Ӧ��Ӱ��
����2��________________
�����ʵ�鷽��������ʵ�顣
������±���ʵ�鲽���Լ�Ԥ������ͽ��ۡ�
��ѡʵ���Լ��� ŨH2SO4��NaCl���塢MnO2���塢ϡ����

��3����Cװ�ó����������к���CO2��HCl��д��C�з�Ӧ�Ļ�ѧ����ʽ______________
ʵ�������ˮ����ȡ������
���ϱ�����������ˮ��ʯ��ʯ��Ӧ����ȡ��ŨHClO��Һ�ķ���֮һ��С���ͬѧ�ݴ���������ʵ�飺
ʵ�������ˮ����ȡ������
���ϱ�����������ˮ��ʯ��ʯ��Ӧ����ȡ��ŨHClO��Һ�ķ���֮һ��С���ͬѧ�ݴ���������ʵ�飺

�����Թ��м�������Ŀ�״̼��ƣ��ټ���Լ20mL������ˮ����ַ�Ӧ�����������ݲ�������Һdz����ɫ��ȥ��
�ڹ��ˣ�����Һ������ɫ�����ϣ����������ˮ��Ư���Ը�ǿ��
��Ϊ��ȷ����Ӧ�������Һ�ֳ����ݣ��ֱ��������ʵ�飺
��һ����ʯ��ˮ��ϣ���������������ɫ������
�ڶ�����ϡ�����ϣ���������������ɫ���壻
�����ݼ��ȣ�������Һ��������д�����ɫ���������
����⣬����ʵ���в�������ɫ�����ΪCO2��
��4��ʵ������õ���ҺƯ������ǿ��ԭ����_____________________
��5����������ʵ�����֪��ʵ��ڵ���Һ�е����ʳ�CaCl2��HClO�⣬������________��
�ڹ��ˣ�����Һ������ɫ�����ϣ����������ˮ��Ư���Ը�ǿ��
��Ϊ��ȷ����Ӧ�������Һ�ֳ����ݣ��ֱ��������ʵ�飺
��һ����ʯ��ˮ��ϣ���������������ɫ������
�ڶ�����ϡ�����ϣ���������������ɫ���壻
�����ݼ��ȣ�������Һ��������д�����ɫ���������
����⣬����ʵ���в�������ɫ�����ΪCO2��
��4��ʵ������õ���ҺƯ������ǿ��ԭ����_____________________
��5����������ʵ�����֪��ʵ��ڵ���Һ�е����ʳ�CaCl2��HClO�⣬������________��
��1��Բ����ƿ����Һ©��
��2���ټ��裺 H+��Ũ�ȶԷ�Ӧ������Ӱ��
��ʵ��
��2���ټ��裺 H+��Ũ�ȶԷ�Ӧ������Ӱ��
��ʵ��

��3��2Cl2+2H2O+C 4HCl+CO2
4HCl+CO2
��4��CaCO3��������ˮ�е�HCl��ʹCl2+H2O HCl+HClOƽ�������ƶ���HClOŨ������
HCl+HClOƽ�������ƶ���HClOŨ������
��5��Ca(HCO3)2
 4HCl+CO2
4HCl+CO2 ��4��CaCO3��������ˮ�е�HCl��ʹCl2+H2O
 HCl+HClOƽ�������ƶ���HClOŨ������
HCl+HClOƽ�������ƶ���HClOŨ��������5��Ca(HCO3)2

��ϰ��ϵ�д�
�����Ŀ
 ijУ��ѧ�о�ѧϰС���������ʵ�鷽�����ⶨС�մ���Ʒ�д��������������
ijУ��ѧ�о�ѧϰС���������ʵ�鷽�����ⶨС�մ���Ʒ�д��������������


