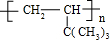��Ŀ����
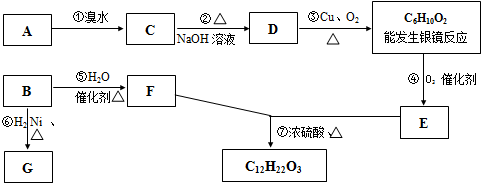
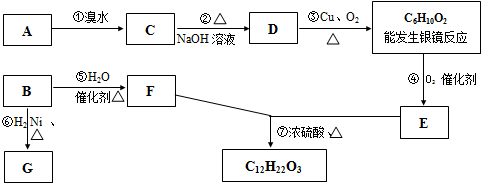
�л��C6H12���������칹��A��B������һЩ�л���֮������ͼ��ʾ��ת����ϵ������G��һ��ȡ����ֻ�����֣�A�ķ��ӽṹ����3������Bת��ΪFʱֻ��һ�ֲ��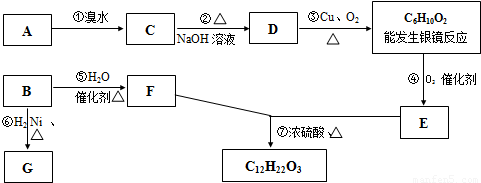
����д���пհף�
��1������ת�������У����ڼӳɷ�Ӧ����______������ţ�
��2���л���G������Ϊ______��
��3���л���A��C12H22O3�ṹ��ʽ�ֱ�Ϊ��A______��C12H22O3______��
��4��д���ڡ��ۡ�������Ӧ�Ļ�ѧ����ʽ����______
���𰸡�������B�����������ӳɷ�Ӧ����G��G��һ��ȡ����ֻ�����֣�����G�Ľṹ��ʽΪ��CH3��2CHCH��CH3��2��B��ˮ�����ӳɷ�Ӧ���ɴ���Bת��ΪFʱֻ��һ�ֲ������B�Ľṹ��ʽΪ��CH3��2C=C��CH3��2��F�Ľṹ��ʽΪ����CH3��2CHC��OH����CH3��2��A�ķ��ӽṹ����3������A�ķ���ʽ��C6H12��A����ˮ�����ӳɷ�Ӧ����±����C��±�������������Ƶ�ˮ��Һ����ȡ����Ӧ���ɴ�D��D���ǻ��������������������ܷ���������Ӧ������A�е�˫���ڱ��ϣ���A�Ľṹ��ʽΪ��CH2=CHC��CH3��3��C�Ľṹ��ʽΪCH2BrCHBrC��CH3��3��D�Ľṹ��ʽΪ��CH2OHCH��OH��C��CH3��3��
D����������C6H10O2�Ľṹ��ʽΪHOCCOC��CH3��3��HOCCOC��CH3��3��������������E��E�Ľṹ��ʽΪ��
HOOCCOC��CH3��3��E��F����������Ӧ��
����⣺B�����������ӳɷ�Ӧ����G��G��һ��ȡ����ֻ�����֣�����G�Ľṹ��ʽΪ��CH3��2CHCH��CH3��2��B��ˮ�����ӳɷ�Ӧ���ɴ���Bת��ΪFʱֻ��һ�ֲ������B�Ľṹ��ʽΪ��CH3��2C=C��CH3��2��F�Ľṹ��ʽΪ����CH3��2CHC��OH����CH3��2��A�ķ��ӽṹ����3������A�ķ���ʽ��C6H12��A����ˮ�����ӳɷ�Ӧ����±����C��±�������������Ƶ�ˮ��Һ����ȡ����Ӧ���ɴ�D��D���ǻ��������������������ܷ���������Ӧ������A�е�˫���ڱ��ϣ���A�Ľṹ��ʽΪ��CH2=CHC��CH3��3��C�Ľṹ��ʽΪCH2BrCHBrC��CH3��3��D�Ľṹ��ʽΪ��CH2OHCH��OH��C��CH3��3��
D����������C6H10O2�Ľṹ��ʽΪHOCCOC��CH3��3��HOCCOC��CH3��3��������������E��E�Ľṹ��ʽΪ��
HOOCCOC��CH3��3��E��F����������Ӧ��
��1��ͨ�����Ϸ���֪�����ڼӳɷ�Ӧ���Ǣ٢ݢޣ��ʴ�Ϊ���٢ݢޣ�
��2��B�������ӳ�����G��G�Ľṹ��ʽΪ��CH3��2CHCH��CH3��2����������2��3-�������飬
�ʴ�Ϊ��2��3-�������飻
��3��ͨ�����Ϸ���֪��A�Ľṹ��ʽΪ����CH3��3CCH=CH2��E��F����������Ӧ����C12H22O3������C12H22O3�Ľṹ��ʽΪ����CH3��3CCOCOOC��CH3��2CH��CH3��2��
�ʴ�Ϊ����CH3��3CCH=CH2����CH3��3CCOCOOC��CH3��2CH��CH3��2��
��4��������������������Ƶ�ˮ��Һ����ȡ����Ӧ���ɴ����廯�⣬���Ǵ���������������ȩͪ������
�ڡ��۷�Ӧ����ʽ�ֱ��ǣ���CH3��3CCHBrCH2Br+2H2O ��CH3��3CCH��OH��CH2OH+2HBr��
��CH3��3CCH��OH��CH2OH+2HBr��
 ��
��
�ʴ�Ϊ����CH3��3CCHBrCH2Br+2H2O ��CH3��3CCH��OH��CH2OH+2HBr��
��CH3��3CCH��OH��CH2OH+2HBr�� ��
��
��5��A��CH2=CHC��CH3��3��һ�������£�A�����Ӿ۷�Ӧ���ɾۺ����Ӧ����ʽΪ��n��CH3��3CCH=CH2
 ���ʴ�Ϊ��n��CH3��3CCH=CH2
���ʴ�Ϊ��n��CH3��3CCH=CH2
 ��
��
���������⿼���л�����ƶ���ϳɣ�����G�Ľṹ�ص��ƶ�G�Ľṹ�ǽ���Ĺؼ���ע�����ת��������Ϲ����ŵ�ת�������ƶ��������ʣ��Ѷ��еȣ��Ƕ��л�֪ʶ���ۺϿ��飬ע�����չ����ŵ�������ת����
D����������C6H10O2�Ľṹ��ʽΪHOCCOC��CH3��3��HOCCOC��CH3��3��������������E��E�Ľṹ��ʽΪ��
HOOCCOC��CH3��3��E��F����������Ӧ��
����⣺B�����������ӳɷ�Ӧ����G��G��һ��ȡ����ֻ�����֣�����G�Ľṹ��ʽΪ��CH3��2CHCH��CH3��2��B��ˮ�����ӳɷ�Ӧ���ɴ���Bת��ΪFʱֻ��һ�ֲ������B�Ľṹ��ʽΪ��CH3��2C=C��CH3��2��F�Ľṹ��ʽΪ����CH3��2CHC��OH����CH3��2��A�ķ��ӽṹ����3������A�ķ���ʽ��C6H12��A����ˮ�����ӳɷ�Ӧ����±����C��±�������������Ƶ�ˮ��Һ����ȡ����Ӧ���ɴ�D��D���ǻ��������������������ܷ���������Ӧ������A�е�˫���ڱ��ϣ���A�Ľṹ��ʽΪ��CH2=CHC��CH3��3��C�Ľṹ��ʽΪCH2BrCHBrC��CH3��3��D�Ľṹ��ʽΪ��CH2OHCH��OH��C��CH3��3��
D����������C6H10O2�Ľṹ��ʽΪHOCCOC��CH3��3��HOCCOC��CH3��3��������������E��E�Ľṹ��ʽΪ��
HOOCCOC��CH3��3��E��F����������Ӧ��
��1��ͨ�����Ϸ���֪�����ڼӳɷ�Ӧ���Ǣ٢ݢޣ��ʴ�Ϊ���٢ݢޣ�
��2��B�������ӳ�����G��G�Ľṹ��ʽΪ��CH3��2CHCH��CH3��2����������2��3-�������飬
�ʴ�Ϊ��2��3-�������飻
��3��ͨ�����Ϸ���֪��A�Ľṹ��ʽΪ����CH3��3CCH=CH2��E��F����������Ӧ����C12H22O3������C12H22O3�Ľṹ��ʽΪ����CH3��3CCOCOOC��CH3��2CH��CH3��2��
�ʴ�Ϊ����CH3��3CCH=CH2����CH3��3CCOCOOC��CH3��2CH��CH3��2��
��4��������������������Ƶ�ˮ��Һ����ȡ����Ӧ���ɴ����廯�⣬���Ǵ���������������ȩͪ������
�ڡ��۷�Ӧ����ʽ�ֱ��ǣ���CH3��3CCHBrCH2Br+2H2O
 ��CH3��3CCH��OH��CH2OH+2HBr��
��CH3��3CCH��OH��CH2OH+2HBr�� ��
���ʴ�Ϊ����CH3��3CCHBrCH2Br+2H2O
 ��CH3��3CCH��OH��CH2OH+2HBr��
��CH3��3CCH��OH��CH2OH+2HBr�� ��
�� ��5��A��CH2=CHC��CH3��3��һ�������£�A�����Ӿ۷�Ӧ���ɾۺ����Ӧ����ʽΪ��n��CH3��3CCH=CH2

 ���ʴ�Ϊ��n��CH3��3CCH=CH2
���ʴ�Ϊ��n��CH3��3CCH=CH2
 ��
�����������⿼���л�����ƶ���ϳɣ�����G�Ľṹ�ص��ƶ�G�Ľṹ�ǽ���Ĺؼ���ע�����ת��������Ϲ����ŵ�ת�������ƶ��������ʣ��Ѷ��еȣ��Ƕ��л�֪ʶ���ۺϿ��飬ע�����չ����ŵ�������ת����

��ϰ��ϵ�д�
�����Ŀ