��Ŀ����
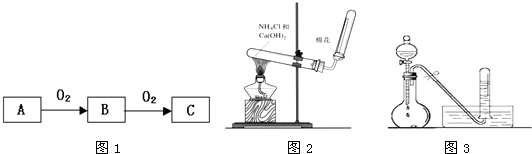
��10�֣�A��B��C����ѧ��ѧ�����ĵ��ʣ�����A�ǽ������ס��ҡ����������������ֻ������������ͼ��ת����ϵ�����ǹ�ҵ����ȡA����Ҫԭ�ϡ�
��1��д���������ʵĻ�ѧʽ��
A ���� ��
��2��д���������ڹ�ҵ�ϵ�����һ����Ҫ��; ��
��3��д�����б仯�ķ���ʽ��
��A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ
�ڹ�ҵ���ü��Ʊ�A�Ļ�ѧ����ʽ
���������CO2��Ӧ�����ӷ���ʽ
��1��Al (1��) AlCl3(1��)
��2��������۱���ʯ���������Ƶ�������Al2O3�����ͻ���ϻ������Ƴ�ɰ�֡���ĥֽ���챦ʯ������ʯ�������������ֱ�����С�(2��)
��3����3����2Al��2NaOH��2H2O��2NaAlO2��3H2��(2��)
��Al2O3 4Al+3O2��(2��)
4Al+3O2��(2��)
��AlO2����CO2�� 2H2O��Al(OH)3����HCO3��(2��)
��������
�������������AΪAl��B��O2��C��H2���ף�Al2O3���ң�NaAlO2����:H2O������Al(OH)3���죺AlCl3��
��2��������ΪAl2O3���������ȷ�Ӧ�����ȼ���Ҳ�����ͻ���ϡ�
��3������ʽ�������ӷ���ʽ������д��Ӧע��������Դ�С�Է�Ӧ��Ӱ�졣��ۣ������CO2��Ӧ�����ʱ������ӦΪHCO3-��
���㣺�����仯���������
�������������ƶ������ʽ��������Ԫ���Լ��������������ʣ����ڻ����⡣�����ƶ���Ľ��Ӧ�ҳ���ͻ�ƿڣ�Ȼ������ͻ�ƿڡ�˳�����ϡ������ɽ��������ʵĽṹ�Ƴ���

 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�


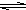 HS-+OH-
HS-+OH- Al��OH��3+3H+����ɫ����Ͱ�ɫ��״��������
Al��OH��3+3H+����ɫ����Ͱ�ɫ��״�������� ��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ��������������ת����ϵ��
��֪A��B��C����ѧ��ѧ�ij������ʣ�������һ��������������ת����ϵ��