��Ŀ����
����A��B��C��D��E����Ԫ�أ����ǵ�ԭ��������������Aԭ���������4�����ӣ�B�������Ӻ�C�������Ӹ������ӵĵ��Ӳ�ṹ��ͬ��Eԭ�ӵ�M���Ӳ��ϵĵ������ȴ������1�����³�ѹ�£�B�ĵ��������壬0.1mol����B��������ȫ��Ӧʱ����2.408��1023�����ӷ���ת�ƣ�C�ĵ����ڸ�������B�ĵ��ʳ�ַ�Ӧ�� ���ɵ���ɫ����F��F��AB��Ӧ������ɵ���B��D���⻯��Ļ�ѧʽ��H2D��D ������������У�����40����D������Dԭ��������ͬ��Ŀ�����ӡ����ӡ�������ɡ��������������ƶϣ���1����Ԫ�صķ��ż�������A________��B________��C________��D________��E________��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
B��C��Ӧ��________��
F��AB2��Ӧ��________��
�𰸣�
������
������
��1��C̼ O�� Na�� S�� Cl��
��2��2Na��O2
|

��ϰ��ϵ�д�
 ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д� ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�
�����Ŀ


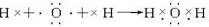



 NH4����OH�������ж�����ˮ���γɵĺ����ṹ��________��(����ͼ�е���ĸ)��
NH4����OH�������ж�����ˮ���γɵĺ����ṹ��________��(����ͼ�е���ĸ)�� 