��Ŀ����
��֪A��B��C��D��Ϊ���壬��������A��BΪ������A���峣���³ʻ���ɫ��A��B��ȼ�յĻ���ʲ�ɫ��D�����ˮ��Һ�ʼ��ԣ�F��Һ�׳�ʯ��ˮ����Ӧ���������������д������̣�GΪ�Ȼ��ƣ�����֮���ת����ϵ��ͼ��ʾ��

��1��д��A��B��D�Ļ�ѧʽ��A______��B______��D______��������D��H����10���ӣ�д��ͬ��10���ӵ������ӻ�ѧʽ______��
��2��D����ˮ��Һ�ĵ��뷽��ʽ��______��E��ˮ�ⷴӦ���ӷ���ʽ��______��
��3���ڷ�Ӧ�١����У�����������ԭ��Ӧ����______��
��4����Ӧ�ڵĻ�ѧ����ʽΪ��______����Ӧ�۵Ļ�ѧ����ʽΪ��______��
�⣺A���峣���³ʻ���ɫ����AΪCl2��A��B��ȼ�յĻ���ʲ�ɫ��BΪH2��CΪHCl��D�����ˮ��Һ�ʼ��ԣ���DΪNH3������EΪNH4Cl��F��Һ�׳�ʯ��ˮ����FΪCa��OH��2��GΪ�Ȼ��ƣ���HΪˮ��
��1��������������֪��AΪCl2��BΪH2��DΪNH3��ͬ��10���ӵ������ӻ�ѧʽ�ֱ�ΪNH4+��H3O+���ʴ�Ϊ��Cl2��H2��NH3��NH4+��H3O+��
��2��D����ˮ��Һ�ĵ��뷽��ʽΪNH3��H2O?NH4++OH-��E��ˮ�ⷴӦ���ӷ���ʽΪNH4++H2O?NH3��H2O+H+��
�ʴ�Ϊ��NH3��H2O?NH4++OH-��NH4++H2O?NH3��H2O+H+��
��3����Ӧ��ΪCl2+H2�T2HCl����Ӧ��ΪHCl+NH3�TNH4Cl����Ӧ��ΪCa��OH��2+2NH4Cl CaCl2+2NH3��+2H2O��ֻ�з�Ӧ������Ԫ�ػ��ϼ۱仯������������ԭ��Ӧ��
CaCl2+2NH3��+2H2O��ֻ�з�Ӧ������Ԫ�ػ��ϼ۱仯������������ԭ��Ӧ��
�ʴ�Ϊ���٣�
��4����Ӧ��ΪHCl+NH3�TNH4Cl����Ӧ��ΪCa��OH��2+2NH4Cl CaCl2+2NH3��+2H2O���ʴ�Ϊ��HCl+NH3�TNH4Cl��Ca��OH��2+2NH4Cl
CaCl2+2NH3��+2H2O���ʴ�Ϊ��HCl+NH3�TNH4Cl��Ca��OH��2+2NH4Cl CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
������A���峣���³ʻ���ɫ����AΪCl2��A��B��ȼ�յĻ���ʲ�ɫ��BΪH2��CΪHCl��D�����ˮ��Һ�ʼ��ԣ���DΪNH3������EΪNH4Cl��F��Һ�׳�ʯ��ˮ����FΪCa��OH��2��GΪ�Ȼ��ƣ���HΪˮ��Ȼ����Ԫ�ػ��������ʼ���ѧ���������
���������⿼��������ƶϣ�ע��������Ϣ�ƶϸ������ǽ��Ĺؼ�����ȷ���������ʼ��������Ʒ����ɽ����ɫΪ����ͻ�ƿڣ���Ŀ�Ѷ��еȣ�
��1��������������֪��AΪCl2��BΪH2��DΪNH3��ͬ��10���ӵ������ӻ�ѧʽ�ֱ�ΪNH4+��H3O+���ʴ�Ϊ��Cl2��H2��NH3��NH4+��H3O+��
��2��D����ˮ��Һ�ĵ��뷽��ʽΪNH3��H2O?NH4++OH-��E��ˮ�ⷴӦ���ӷ���ʽΪNH4++H2O?NH3��H2O+H+��
�ʴ�Ϊ��NH3��H2O?NH4++OH-��NH4++H2O?NH3��H2O+H+��
��3����Ӧ��ΪCl2+H2�T2HCl����Ӧ��ΪHCl+NH3�TNH4Cl����Ӧ��ΪCa��OH��2+2NH4Cl
 CaCl2+2NH3��+2H2O��ֻ�з�Ӧ������Ԫ�ػ��ϼ۱仯������������ԭ��Ӧ��
CaCl2+2NH3��+2H2O��ֻ�з�Ӧ������Ԫ�ػ��ϼ۱仯������������ԭ��Ӧ���ʴ�Ϊ���٣�
��4����Ӧ��ΪHCl+NH3�TNH4Cl����Ӧ��ΪCa��OH��2+2NH4Cl
 CaCl2+2NH3��+2H2O���ʴ�Ϊ��HCl+NH3�TNH4Cl��Ca��OH��2+2NH4Cl
CaCl2+2NH3��+2H2O���ʴ�Ϊ��HCl+NH3�TNH4Cl��Ca��OH��2+2NH4Cl CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��������A���峣���³ʻ���ɫ����AΪCl2��A��B��ȼ�յĻ���ʲ�ɫ��BΪH2��CΪHCl��D�����ˮ��Һ�ʼ��ԣ���DΪNH3������EΪNH4Cl��F��Һ�׳�ʯ��ˮ����FΪCa��OH��2��GΪ�Ȼ��ƣ���HΪˮ��Ȼ����Ԫ�ػ��������ʼ���ѧ���������
���������⿼��������ƶϣ�ע��������Ϣ�ƶϸ������ǽ��Ĺؼ�����ȷ���������ʼ��������Ʒ����ɽ����ɫΪ����ͻ�ƿڣ���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ




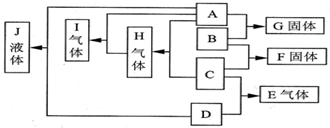

 ��֪A��B��C��D��Ϊ���壬E��F��Ϊ�����³ʹ�������ӻ����GΪ�Ȼ��ƣ�A��B��ȼ�յĻ���ʲ�ɫ����Ӧ���������������д������̣�����֮���ת����ϵ��ͼ��ʾ��
��֪A��B��C��D��Ϊ���壬E��F��Ϊ�����³ʹ�������ӻ����GΪ�Ȼ��ƣ�A��B��ȼ�յĻ���ʲ�ɫ����Ӧ���������������д������̣�����֮���ת����ϵ��ͼ��ʾ��


