��Ŀ����
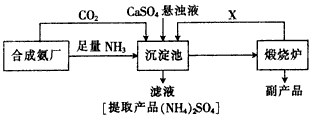
�ϳɰ���ҵ�IJ��ֹ�����������ͼ��ʾ��

����ش��������⣺
��1����֪��N2(g)+O2(g) =2NO(g)����H=180.5kJ��mol-1
4NH3(g)+5O2(g)=4NO(g)+6H2O(g) ����H=�C905kJ��mol-1
2H2(g)+O2(g)=2H2O(g) ����H=�C483.6kJ��mol-1
��N2��g��+3H2��g�� 2NH3��g���ġ�H=_________________��
2NH3��g���ġ�H=_________________��
��2���ı�������������ʹƽ��������Ӧ���������ƽ�ⳣ���������_________��
A.����ѹǿ B.�����¶� C.ʹ�ô��� D. ����Ӧ���Ũ��
��3����һ�������£���2molN2��5molH2�����һ��10L���ܱ������У���Ӧ�������ͼ��ʾ��
����5min�ڵ�ƽ����Ӧ����v(NH3)=_______________
�ڴﵽƽ��ʱNH3���������Ϊ___________%
(4)��������ѧ������˵��ϳɰ��ķ��������ø����ӵ����Ե�SCY�մɣ��ܴ���H+��Ϊ���ʣ�������������������ϵĽ����ٶྦྷ��Ĥ���缫��ʵ���˸�ת���ʵĵ�ⷨ�ϳɰ���װ����ͼ��

��ش��ٵ缫A�ǵ��ص�_______��������������������ü��ϵĵ缫��Ӧʽ��_________________________
��1�� ��92.4kJ��mol-1
��2�� AD
��3��0.04mol/(L��min) 40
(4) �� ; N2 + 6e��+6H+= 2NH3
��������

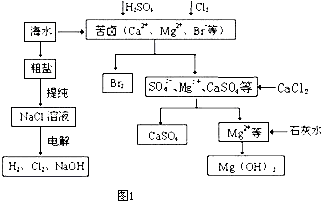
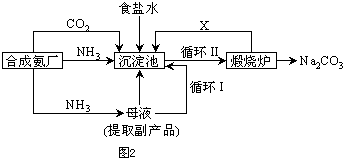
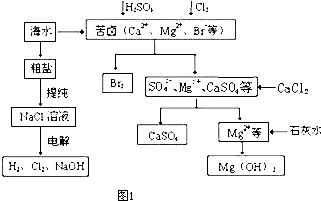
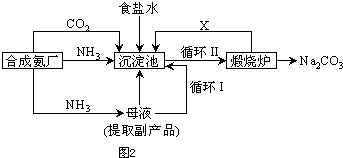
 A��ʳ�εı�����Һ
A��ʳ�εı�����Һ ����Һ
����Һ ����
���� ����
����


 CO2+H2
CO2+H2