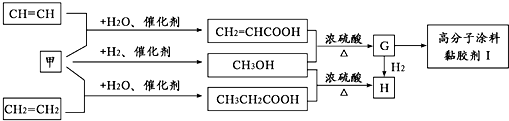
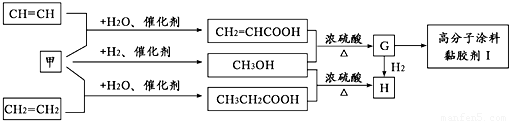
��Ŀ����
��֪A��B��C���Ƕ�����Ԫ�أ�A�Ļ�����ͨ��Ϊ+1��A��B���γ�A2B��A2B2�����ֻ����A2B��������10�����ӣ�A��C�γɵ�һ�ֻ��������л����к���������������ģ�B��C����Ԫ���γɵ�һ�ֳ���������ף������Ǽ���ú������Ҫ�ɷ֣�Ҳ����Ҫ�Ļ���ԭ�ϣ���ͼ����ijЩ���л����ڵ��¡���ѹ�ʹ��������ºϳɾ����������ܵ�װ���Ը߷���Ϳ�ϡ�𤽺��I�Ļ������̣�

�ݴˣ���ش��������⣺
��1��д���������ʵķ���ʽ��ṹ��ʽ��
A2B2����ʽ
 ��
��
��2��д�����б仯�Ļ�ѧ����ʽ��
��+H2��CH3OH���״���

�ݴˣ���ش��������⣺
��1��д���������ʵķ���ʽ��ṹ��ʽ��
A2B2����ʽ
H2O2
H2O2
���ķ���ʽCO
CO
��I�Ľṹ��ʽ

��2��д�����б仯�Ļ�ѧ����ʽ��
��+H2��CH3OH���״���
CO+2H2
CH3OH
| ���� |
CO+2H2
CH3OH
��G+H2��H| ���� |
CH2=CHCOOCH3+H2
CH3CH2COOCH3
| ���� |
CH2=CHCOOCH3+H2
CH3CH2COOCH3
��| ���� |
������A��C�γɵ�һ�ֻ��������л����к���������������ģ��������Ǽ��飬A�Ļ�����ͨ��Ϊ+1����AΪH��CΪC��A��B���γ�A2B��A2B2�����ֻ����A2B��������10�����ӣ���BΪO����A2B��A2B2�ֱ�ΪH2O��H2O2��B��C����Ԫ���γɵ�һ�ֳ���������ף������Ǽ���ú������Ҫ�ɷ֣�Ҳ����Ҫ�Ļ���ԭ�ϣ��ʼ�ΪCO��CH2=CHCOOH��״���Ӧ����G��GΪCH2=CHCOOCH3��������״���Ӧ����H��HΪCH3CH2COOCH3��G��һ������������I��IΪ ���ٽ�϶�Ӧ�л���Ľṹ�����ʣ��Լ�ǰ��ת����ϵ�����⣮
���ٽ�϶�Ӧ�л���Ľṹ�����ʣ��Լ�ǰ��ת����ϵ�����⣮
 ���ٽ�϶�Ӧ�л���Ľṹ�����ʣ��Լ�ǰ��ת����ϵ�����⣮
���ٽ�϶�Ӧ�л���Ľṹ�����ʣ��Լ�ǰ��ת����ϵ�����⣮����⣺��1��A��C�γɵ�һ�ֻ��������л����к���������������ģ��������Ǽ��飬A�Ļ�����ͨ��Ϊ+1����AΪH��CΪC��A��B���γ�A2B��A2B2�����ֻ����A2B��������10�����ӣ���B����8�����ӣ���BΪO����A2B��A2B2�ֱ�ΪH2O��H2O2��B��C����Ԫ���γɵ�һ�ֳ���������ף������Ǽ���ú������Ҫ�ɷ֣�Ҳ����Ҫ�Ļ���ԭ�ϣ��ʼ�ΪCO��CH2=CHCOOH��״���Ӧ����G��GΪCH2=CHCOOCH3��G��һ������������I��
IΪ ���ʴ�Ϊ��H2O2��CO��
���ʴ�Ϊ��H2O2��CO�� ��
��
��2��CO��������Ӧ���ɼ״�������ʽΪ��CO+2H2
CH3OH��CH2=CHCOOCH3�����������ӳɷ�Ӧ������ʽΪCH2=CHCOOCH3+H2
CH3CH2COOCH3��
�ʴ�Ϊ��CO+2H2
CH3OH��CH2=CHCOOCH3+H2
CH3CH2COOCH3��
IΪ
 ���ʴ�Ϊ��H2O2��CO��
���ʴ�Ϊ��H2O2��CO�� ��
����2��CO��������Ӧ���ɼ״�������ʽΪ��CO+2H2
| ���� |
| ���� |
�ʴ�Ϊ��CO+2H2
| ���� |
| ���� |
�������л��ƶ��Ǹ߿��ȵ����ͣ�ÿ��߿��ؿ�����ѧ�����Ķ���˼ά����Ҫ��ϸߣ��л���Ӧ����ʽ����д�ǹؼ����ر��ǾۺϷ�Ӧ�����ʽϸߣ�

��ϰ��ϵ�д�
�����Ŀ