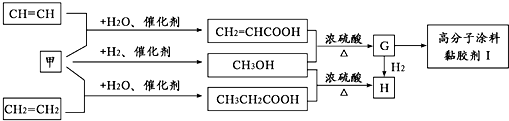
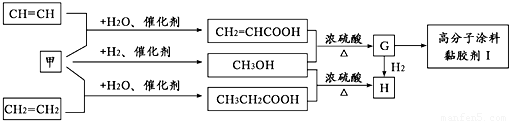
��Ŀ����
��֪A��B��C���Ƕ�����Ԫ�أ�A�Ļ�����ͨ��Ϊ+1��A��B���γ�A2B��A2B2�����ֻ����A2B��������10�����ӣ�A��C�γɵ�һ�ֻ��������л����к���������������ģ�B��C����Ԫ���γɵ�һ�ֳ���������ף������Ǽ���ú������Ҫ�ɷ֣�Ҳ����Ҫ�Ļ���ԭ�ϣ���ͼ����ijЩ���л����ڵ��¡���ѹ�ʹ��������ºϳɾ����������ܵ�װ���Ը߷���Ϳ�ϡ�𤽺��I�Ļ������̣�

�ݴˣ���ش��������⣺
��1��д���������ʵķ���ʽ��ṹ��ʽ��
A2B2����ʽ
 ��
��
��2��C��һ�ֵ��ʵľ����в����ڵ������ӣ��þ���Ҳ�����磬������
��3��д�����б仯�Ļ�ѧ����ʽ����+H2��CH3OH���״���

�ݴˣ���ش��������⣺
��1��д���������ʵķ���ʽ��ṹ��ʽ��
A2B2����ʽ
H2O2
H2O2
���ķ���ʽCO
CO
��I�Ľṹ��ʽ

��2��C��һ�ֵ��ʵľ����в����ڵ������ӣ��þ���Ҳ�����磬������
ԭ��
ԭ��
���壮��3��д�����б仯�Ļ�ѧ����ʽ����+H2��CH3OH���״���
CO+2H2
CH3OH
| ���� |
CO+2H2
CH3OH
��G+H2��H| ���� |
CH2=CHCOOCH3+H2
CH3CH2COOCH3
| ���� |
CH2=CHCOOCH3+H2
CH3CH2COOCH3
��| ���� |
��������֪A��B��C���Ƕ�����Ԫ�أ�A�Ļ�����ͨ��Ϊ+1��A��B���γ�A2B��A2B2�����ֻ����A2B��������10�����ӣ���֪AΪ��Ԫ�ء�BΪ��Ԫ�أ��л����к���������������ļ��飬��֪CΪ̼Ԫ�أ�B��C����Ԫ���γɵ�һ�ֳ���������ף������Ǽ���ú������Ҫ�ɷ֣�Ҳ����Ҫ�Ļ���ԭ�ϣ���֪��Ϊһ����̼��
��1������A2B2Ϊ�������⣻��Ϊһ����̼������CH2=CHCO0H��HOCH3����������Ӧ����CH2=CHCOOCH3��CH2=CHCOOCH3�к���̼̼˫�����ܹ������Ӿ۷�Ӧ��
��2��C��һ�ֵ��ʵľ����в����ڵ������ӣ��þ���Ҳ�����磬�õ���Ϊ���ʯ��
��3������һ����̼�����������˼״�������CH2=CHCO0H��HOCH3����������Ӧ����CH2=CHCOOCH3��CH2=CHCOOCH3�������������ӳɷ�Ӧ��
��1������A2B2Ϊ�������⣻��Ϊһ����̼������CH2=CHCO0H��HOCH3����������Ӧ����CH2=CHCOOCH3��CH2=CHCOOCH3�к���̼̼˫�����ܹ������Ӿ۷�Ӧ��
��2��C��һ�ֵ��ʵľ����в����ڵ������ӣ��þ���Ҳ�����磬�õ���Ϊ���ʯ��
��3������һ����̼�����������˼״�������CH2=CHCO0H��HOCH3����������Ӧ����CH2=CHCOOCH3��CH2=CHCOOCH3�������������ӳɷ�Ӧ��
����⣺��1������A2B2Ϊ�������⣬����ʽΪ��H2O2����Ϊһ����̼������ʽΪCO������CH2=CHCO0H��HOCH3����������Ӧ����CH2=CHCOOCH3��CH2=CHCOOCH3�к���̼̼˫�����ܹ������Ӿ۷�Ӧ��nCH2=CHCOOCH3
 ��
��
�ʴ�Ϊ��H2O2��CO�� ��
��
��2��C��һ�ֵ��ʵľ����в����ڵ������ӣ��þ���Ҳ�����磬�õ���Ϊ���ʯ����ԭ�Ӿ��壬�ʴ�Ϊ��ԭ�ӣ�
��3��һ����̼�����������˼״�������ʽΪ��CO+2H2
CH3OH��CH2=CHCO0H��HOCH3����������Ӧ����CH2=CHCOOCH3��CH2=CHCOOCH3�������������ӳɷ�Ӧ��CH2=CHCOOCH3+H2
CH3CH2COOCH3��
�ʴ�Ϊ��CO+2H2
CH3OH��CH2=CHCOOCH3+H2
CH3CH2COOCH3��
| ���� |
 ��
���ʴ�Ϊ��H2O2��CO��
 ��
����2��C��һ�ֵ��ʵľ����в����ڵ������ӣ��þ���Ҳ�����磬�õ���Ϊ���ʯ����ԭ�Ӿ��壬�ʴ�Ϊ��ԭ�ӣ�
��3��һ����̼�����������˼״�������ʽΪ��CO+2H2
| ���� |
| ���� |
�ʴ�Ϊ��CO+2H2
| ���� |
| ���� |
���������⿼���Ϊ�ۺϣ��漰Ԫ�ص��ƶϡ��л���ѧ����ʽ����д�����⣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ