��Ŀ����
��10�֣�ijʵ��С����0��50 mol��L��1 NaOH��Һ��0��50 mol��L��1������Һ�����к��ȵIJⶨ��
������0��50 mol��L��1 NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH����________g��
��2������ͼ��ѡ�����NaOH��������Ҫ������(����ĸ)��__________��
�ⶨϡ�����ϡ���������к��ȵ�ʵ��װ������ͼ��ʾ��
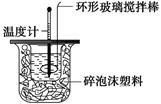
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ(�к���Ϊ57��3 kJ��mol��1)��_________________________
________________________________________________________________________��
��2��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
�ڽ�����Ϊ0��50 mol��L��1 NaOH��Һ��0��50 mol��L��1������Һ���ܶȶ���1 g��cm��3���кͺ�������Һ�ı�����c��4��18 J��g��1������1�����к��Ȧ�H��_______________________(ȡС�����һλ)��
������ʵ����ֵ�����57��3 kJ��mol��1��ƫ�����ƫ���ԭ�������(����ĸ)____________��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
������0��50 mol��L��1 NaOH��Һ
��1����ʵ���д�ԼҪʹ��245 mL NaOH��Һ��������Ҫ����NaOH����________g��
��2������ͼ��ѡ�����NaOH��������Ҫ������(����ĸ)��__________��
| ���� | ������ƽ(������) | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
| ���� |  |  |  |  |  |  |
| ��� | a | b | c | d | e | f |

��1��д���÷�Ӧ���Ȼ�ѧ����ʽ(�к���Ϊ57��3 kJ��mol��1)��_________________________
________________________________________________________________________��
��2��ȡ50 mL NaOH��Һ��30 mL������Һ����ʵ�飬ʵ���������±���
������д�±��еĿհף�
 �¶� �¶�ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ (t2��t1)/�� | ||
| H2SO4 | NaOH | ƽ��ֵ | |||
| 1 | 26��2 | 26��0 | 26��1 | 30��1 | |
| 2 | 27��0 | 27��4 | 27��2 | 31��2 | |
| 3 | 25��9 | 25��9 | 25��9 | 29��8 | |
| 4 | 26��4 | 26��2 | 26��3 | 30��4 | |
������ʵ����ֵ�����57��3 kJ��mol��1��ƫ�����ƫ���ԭ�������(����ĸ)____________��
a��ʵ��װ�ñ��¡�����Ч����
b����ȡNaOH��Һ�����ʱ���Ӷ���
c���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
44����1��5��0��2��abe
��1��H2SO4(aq)��2NaOH(aq)===Na2SO4(aq)��2H2O(l)��H����114��6 kJ��mol��1
��2����4��0�ڣ�53��5 kJ��mol��1��acd
��1��H2SO4(aq)��2NaOH(aq)===Na2SO4(aq)��2H2O(l)��H����114��6 kJ��mol��1
��2����4��0�ڣ�53��5 kJ��mol��1��acd
�����������1��245ml����Һ����Ҫ����250ml����Һ������Ҫ�������250mL��������������Ƶ���������2������������ŵ�С�ձ��г��������õ���ƽ��С�ձ���Կ�ף�II��1���к�����ָ����1molH2O�ų���������������2molH2Oʱ�ų�������Ҳ�ӱ�����2������ֹ�¶ȼ�ȥ��ʼ�¶�ƽ��ֵ�����ÿ�ε��¶Ȳ�����Ĵε�ƽ��ֵ���ڱ����ݳ�����Һ�����������������������1molˮ�ų������������kJ�����к��ȣ���bѡ����ȡ��������ʱ���Ӷ�����ʹ��ȡ��������������ų��������࣬��b����ac������������ʧ������ȷ��d���¶ȼ��ϸ��ŵ��������ƺ����ᷴӦ�ˣ��ⲿ��������ʧ�ˣ���ѡacd��

��ϰ��ϵ�д�
�����Ŀ






 6H2O����IJ������� ����ȴ�ᾧ�����ˣ��ù����豣�������������ϱ�Ҫ�����ӷ���ʽ˵��ԭ�� .
6H2O����IJ������� ����ȴ�ᾧ�����ˣ��ù����豣�������������ϱ�Ҫ�����ӷ���ʽ˵��ԭ�� .