��Ŀ����
��һ�λ�ѧ�о���ѧϰʵ���У�ij�о�С��ѧ�����о�����ij��ƿ�ޱ�ǩ���Լ���ֻ֪��һƿ����������һƿ��Һ����ȡ���������Լ����Թ��л�ϣ���������һ����ɫ��ζ�����塣������Դ����忪չһϵ��̽�������������ʵ�鱨�档
��1���ٴ�������ʲô���ʣ�ֻд�����п��ܵ�һ�֣�����֤����ʵ�鷽�����£�
| ���� | ʵ�鲽�� | ������ |
| �����������____ | __ |
����������Ļ�ѧ����ʽ������___________________________
������ȡ�����壨�ñ�������ƿ�ޱ�ǩ���Լ��������õķ���װ�ÿ�ѡ����ͼA��B��C�е�__________ (��Ӧ�����)���ռ��������ѡ��D�е� װ�á�


������ͼE���ռ��������װ�ã������� �ˣ��a����b�������롣

E
(2)�������ɫ���廹����������һ�����壬����У�
��д���������Ļ�ѧ����ʽ______ __ ___��ֻ��дһ�֣���
��1��������(O2)[�������̼(CO2)������(H2)]����2�֣��������ǵ�ľ�������Թ��У�ľ����ȼ����֤������������������ͨ������ʯ��ˮ�У�ʯ��ˮ����ǣ���֤���Ƕ�����̼�����Թܿڶ�һ�������ȼ�����壬��ȼ�գ���������ɫ�������������������֤��������������4�֣�2H2O2 ![]() 2H2O +O2������ CaCO3 + 2HCl = CaCl2 + H2O + CO2����Zn + 2HCl = ZnCl2 + H2��������2�֣�
2H2O +O2������ CaCO3 + 2HCl = CaCl2 + H2O + CO2����Zn + 2HCl = ZnCl2 + H2��������2�֣�
�� B�� ��2�֣���������O2��CO2����a��H2����b����2�֣�
��2�����������е���һ�ֵķ���ʽ���ɣ�����ij��⣩����2�֣�

��һ�λ�ѧ�о���ѧϰʵ���У�ij�о�С��ѧ�����о�����ij��ƿ�ޱ�ǩ���Լ���ֻ֪��һƿ�ǹ��壬��һƿ��Һ�壬ȡ���������Լ����Թ��л�ϣ���������һ����ɫ��ζ�����塣������Դ����忪չһϵ��̽�������������ʵ�鱨�档
��1���ٴ�������ʲô���ʣ�ֻд�����п��ܵ�һ�֣�����֤����ʵ�鷽�����£�
���� | ʵ�鲽�� | ������ |
�����������____ | _________________________ ________________________ | ____________ _____________ __ |
����������Ļ�ѧ����ʽ������___________________________.
������ȡ�����壬���õķ���װ�ÿ�ѡ������ͼ�е�__________ (��Ӧ�����)���ռ��������ѡ��D�е�ijһ��װ�á�

������ͼ���ռ��������װ�ã�
������ �ˣ��a����b�������롣
(2)�������ɫ���廹����������һ�����壬����У���д���������Ļ�ѧ����ʽ______ ֻ��дһ�֣���
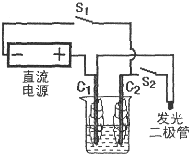 ȼ�ϵ����һ�ֽ���ѧ��Ӧ����������ֱ��ת���ɵ��ܵ�װ�ã�ij�о���ѧϰС����һ�λ�У�������ͼ��װʵ��װ�ã��������������̼��Ϊ�缫���������Һ��0.5mol?L-1Na2SO4��Һ����Դ��3��6Vֱ����Դ�������������Ե�ѹΪ1.7V������Ϊ0.6mA��
ȼ�ϵ����һ�ֽ���ѧ��Ӧ����������ֱ��ת���ɵ��ܵ�װ�ã�ij�о���ѧϰС����һ�λ�У�������ͼ��װʵ��װ�ã��������������̼��Ϊ�缫���������Һ��0.5mol?L-1Na2SO4��Һ����Դ��3��6Vֱ����Դ�������������Ե�ѹΪ1.7V������Ϊ0.6mA��


