��Ŀ����
����Ŀ����Ȳ��������ϩ��ҵ�е���Ҫ���Ʒ�Ӧ��������һ��Ӧ���Խ���ϩ��Ʒ�е���Ȳ�������ͣ��Ա��������ϩ�ۺϴ������ж�����ҵ�ϳ�Ϊ̼��������̡�
��֪����.CH![]() CH(g)+H2(g)��CH2=CH2(g) ��H1 K1(400K)=4.2��1022
CH(g)+H2(g)��CH2=CH2(g) ��H1 K1(400K)=4.2��1022
��.CH![]() CH(g)+2H2(g)��CH3CH3(g) ��H2=-311.4kJ��mol-1 K2(400K)=1.4��1038
CH(g)+2H2(g)��CH3CH3(g) ��H2=-311.4kJ��mol-1 K2(400K)=1.4��1038
�ش��������⣺
��1����֪���ֻ�ѧ���ļ������±���ʾ��

��H1=___kJmol-1��
��2��400Kʱ�����ܱ������н������ʵ�����CH2=CH2(g)��H2(g)��ϣ������ʵ��Ĵ������з�Ӧ������CH3CH3(g)���ﵽƽ��ʱ���![]() =1016����ƽ��ʱc(H2)=___molL-1��
=1016����ƽ��ʱc(H2)=___molL-1��
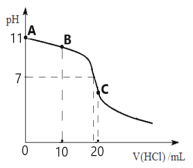
��3����ǰ���о�������Ȳ��PV�Ŵر�������ⷴӦ�IJ���������ͼ1��ʾ������������PV�����ϵ�������*��ע��

�Ʋ���ϩ��PV�����ϵ�����Ϊ___(�������������������������̡�ͼ1����������ܽ𣨻�ܣ�E��=___kJ��mol-1���ò���Ļ�ѧ����ʽΪ___��
��4��T1��ʱ���������Ϊ1��2��CH��CH(g)��H2(g)��������ܱ������У��������������Ӧ����ʼ��ϵ��ѹǿΪP0 kPa��ʵ����H2�ķ�ѹ��p)�뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ2��ʾ��

��T1��ʱ��0��4min�ڣ�ƽ����Ӧ����v(HC��CH)=___kPamin-1(�ú�p0��p1�Ĵ���ʽ��ʾ����ͬ����
��T1��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��Kp=___kPa-2(KpΪ�Է�ѹ��ʾ��ƽ�ⳣ������ѹ=��ѹ�����ʵ�����������
��T1��ʱ��0��2min��p(H2)�ļ�С��___(������������������=����2��4min��p(H2)�ļ�С��������Ϊ___��
���𰸡�-193.8 3 ���� 22.59 C2H3+ H= C2H4+ ![]()
![]() �� ��Ӧ���Ũ�ȼ�С����Ӧ���ʼ���
�� ��Ӧ���Ũ�ȼ�С����Ӧ���ʼ���
��������
(1)��ϱ����м������ݣ����ݡ�H=��Ӧ��ļ��� -������ļ��ܽ��н��
(2)���ø�˹���ɽ��Ŀ�귴Ӧ��������֪��Ӧ��Ŀ�귴Ӧ��ƽ�ⳣ����ϵ���㣻
(3)���ͼ�и����ʷ�Ӧ��ϵ�з�Ӧ�������������仯�����жϣ�
(4)���á�����ʽ���㡱��Ӧ���ʡ�ƽ�ⳣ������Ӧ�����з�Ӧ���Ũ�ȱ仯������
(1)���ݡ�H=��Ӧ��ļ��� -������ļ��ܣ�����H1=413.4 kJmol-1��2+812 kJmol-1+436 kJmol-1-(413.4 kJmol-1��4+615 kJmol-1)=-193.8 kJmol-1��
(2)��֪����.CH![]() CH(g)+H2(g)��CH2=CH2(g) ��H1 K1(400K)=4.2��1022
CH(g)+H2(g)��CH2=CH2(g) ��H1 K1(400K)=4.2��1022
��.CH![]() CH(g)+2H2(g)��CH3CH3(g) ��H2=-311.4kJ��mol-1 K2(400K)=1.4��1038
CH(g)+2H2(g)��CH3CH3(g) ��H2=-311.4kJ��mol-1 K2(400K)=1.4��1038
���ݸ�˹���ɣ���-��ã�CH2=CH2(g)+H2(g)��CH3CH3(g)��ƽ��ʱ���![]() =1016��ƽ�ⳣ��K=
=1016��ƽ�ⳣ��K=![]() =
=![]() =
=![]() ����c(H2)=3mol/L��
����c(H2)=3mol/L��
(3)��ͼ1��ʾ��IM6ΪC2H4��C2H4��C2H4�Ĺ���Ϊ��ϩ���Ѹ����̣���Ҫ����14.58 kJmol-1����������֮C2H4��C2H4�Ĺ���Ϊ��ϩ���������̣��ų�14.58 kJmol-1����������ͼ��ʾ��IM4��IM5�����л�����Ϊ-32.33 kJmol-1-(-54.92 kJmol-1)=22.59 kJmol-1���ò���Ļ�ѧ��Ӧ����ʽΪ��C2H3+ H= C2H4+ ��
(4)�ٸ��ݷ�Ӧ��CH![]() CH(g)+2H2(g)��CH3CH3(g)����ͼ��ʾ����ʼʱp(CH
CH(g)+2H2(g)��CH3CH3(g)����ͼ��ʾ����ʼʱp(CH![]() CH)=
CH)=![]() kPa��p(H2)=
kPa��p(H2)=![]() kPa������������ʽ����
kPa������������ʽ����

��2x=![]() -
-![]() �����x=
�����x=![]() - p1����0-4min�ڣ�ƽ����Ӧ����v(HC��CH)=
- p1����0-4min�ڣ�ƽ����Ӧ����v(HC��CH)= kPamin-1��
kPamin-1��
���ɢٵġ�����ʽ���ɵ�Kp= ��
��
�����ŷ�Ӧ�Ľ��У���Ӧ���Ũ�ȼ�С����Ӧ���ʼ�������0��2min�������ı仯������2��4min�ڣ���0��2min��p(H2)�ļ�С����2��4min��p(H2)�ļ�С����

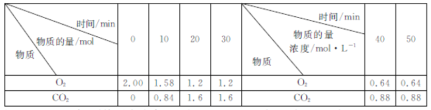
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��CO��H2��ú�����������������������;�㷺��
��1��CO��ԭ������������Ⱦ��SO2
��2CO��g�� + SO2��g�� ![]() S��s��+2CO2��g�� H = -270 kJ��mol��1���÷�Ӧ��ƽ�ⳣ������ʽΪ__��
S��s��+2CO2��g�� H = -270 kJ��mol��1���÷�Ӧ��ƽ�ⳣ������ʽΪ__��
���ھ��Ⱥ��ݵ��ܱ������н���������Ӧ������˵����ȷ����_____��
a ����������ܶȱ��ֲ��䣬���Ѵ�ƽ��״̬
b ��ƽ������ٳ���һ����CO2��ƽ�ⳣ�����ֲ���
c ���������S�������淴Ӧ���ʾ����ֲ��䣬ƽ�ⲻ�ƶ�
d �ӷ�Ӧ��ʼ��ƽ�⣬�����������ѹǿ���ֲ���
����2 L���º����ܱ�������ͨ��2 mol CO��1 mol SO2���ֱ����a��b��c����ʵ�顣�ڲ�ͬ�������·�����Ӧ��2CO��g�� + SO2��g�� ![]() S��s��+2CO2��g�� H = -270 kJ��mol��1����Ӧ��ϵ��ѹ��ʱ��ı仯���±���ʾ��������ʵ���¶ȵĴ�С��ϵ��_____����a��b��c��ʾ����ʵ��a�ӷ�Ӧ��ʼ��45s�ﵽƽ�⣬��ù��̷�Ӧ����v��SO2��__________���������2λ��Ч���֣���
S��s��+2CO2��g�� H = -270 kJ��mol��1����Ӧ��ϵ��ѹ��ʱ��ı仯���±���ʾ��������ʵ���¶ȵĴ�С��ϵ��_____����a��b��c��ʾ����ʵ��a�ӷ�Ӧ��ʼ��45s�ﵽƽ�⣬��ù��̷�Ӧ����v��SO2��__________���������2λ��Ч���֣���
| 0s | 40s | 45s | 60s |
a | 175 | 142 | 140 | 140 |
b | 160 | 120 | 120 | 120 |
c | 160 | 130 | 125 | 120 |
��2������CO��H2���Ʊ���Ȼ������Ҫ��ӦΪ��
CO��g�� + 3H2��g�� ![]() CH4��g�� + H2O��g�� H1=-206.2 kJ��mol1��
CH4��g�� + H2O��g�� H1=-206.2 kJ��mol1��
CO��g�� + H2O��g�� ![]() CO2��g�� + H2��g�� H2 = -41.0 kJ��mol��1��
CO2��g�� + H2��g�� H2 = -41.0 kJ��mol��1��
H2O��l�� �TH2O��g�� H3 =+44 kJ��mol��1 ��
�ش��������⣺
�ٷ�ӦCO2��g�� + 4H2��g�� ![]() CH4��g�� + 2H2O��l�� ��H4 = ________ kJ��mol��1��ij�¶��£��ֱ�����ʼ�ݻ���ͬ�ĺ�ѹ����A����������B�м���1molCO2��4molH2�Ļ�����壬��������Ӧ�ﵽƽ���ų������յ������϶����__�� ����A������B"����
CH4��g�� + 2H2O��l�� ��H4 = ________ kJ��mol��1��ij�¶��£��ֱ�����ʼ�ݻ���ͬ�ĺ�ѹ����A����������B�м���1molCO2��4molH2�Ļ�����壬��������Ӧ�ﵽƽ���ų������յ������϶����__�� ����A������B"����
���ں�ѹ�ܵ���Ӧ���а�n��H2��:n��CO�� = 3:1ͨ��ԭ�������ڴ����������Ʊ��ϳ���Ȼ����400 �� p��Ϊ100 kPaʱ��Ӧ��ϵƽ��������±���ʾ��
��� | CH4 | H2O | H2 | CO2 | CO |
�������/% | 45.0 | 42.5 | 10.0 | 1.50 | 1.00 |
���������CO����ת������=____��![]()
���Ʊ��ϳ���Ȼ��������ԭ������ͨ��ˮ���������������̼��
��̼��ӦΪ����ӦI ��CH4��g�� ![]() C��s�� + 2H2��g�� H = +75 kJ��mol��1��
C��s�� + 2H2��g�� H = +75 kJ��mol��1��
��Ӧ��2CO��g�� ![]() C��s�� + CO2��g�� H = -172 kJ��mol��1��
C��s�� + CO2��g�� H = -172 kJ��mol��1��
ƽ����ϵ��ˮ����Ũ�ȶԻ�̼����Ӱ����ͼ��ʾ������˵����ȷ����__��
A ����1��700 ~ 800���̼����С��ԭ������Ƿ�Ӧ�������ƶ�
B ����1��550 ~700���̼�������ԭ������Ƿ�ӦI�������������
C ����2��3��550 ~800���̼���ϵ͵�ԭ����ˮ����ϡ������ʹ��̼��Ӧ���ʼ�С
D ˮ���������շ�Ӧ�ų���������������ϵ�¶���550�����£������ڼ��ٻ�̼