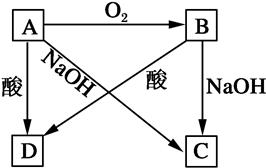
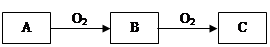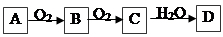��Ŀ����
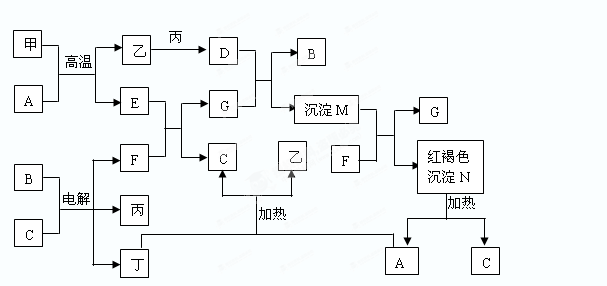
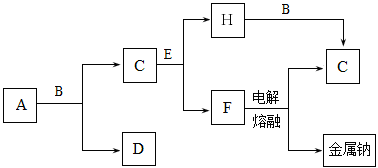
A��B��C��D��E��F�dz������ʣ�����A���������Ӧ����㷺�Ľ�����Ԫ��D�ǵؿ��к������Ľ���Ԫ�أ�DԪ�غ�EԪ�������ڱ������ڡ�G��H��I��J��K��L�dz������������G�ڳ���������ɫҺ�壬K��L����ɫ���壬��K�ǷǼ��Է��ӣ�F��H�Ǻ�ɫ���塣������������ת����ϵ����ͼ��ʾ��
��ش��������⣺
��1��Ԫ��A�ĺ�������Ų�ʽΪ________________��
��2��D��������������Һ��Ӧ����д���÷�Ӧ�����ӷ���ʽ___________________��
��3��д��A��G��Ӧ�Ļ�ѧ����ʽ_______________________________________��
��4��д��H��D��Ӧ�Ļ�ѧ����ʽ______________________________________��
��5��д��K��E��Ӧ�Ļ�ѧ����ʽ_________________________________________��
��1��1s22s22p63s23p63d64s2��[Ar] 3d64s2
��2��2Al + 2OH- + 6H2O = 2 [Al(OH)4]-+ 3H2��  ��3��3Fe + 4H2O(g ) Fe3O4 + 4H2
��3��3Fe + 4H2O(g ) Fe3O4 + 4H2 ��4��8Al + 3Fe3O4 4Al2O3 + 9Fe
��4��8Al + 3Fe3O4 4Al2O3 + 9Fe
��5��2Mg + CO2  2MgO + C ������ʽÿ����2�֣�
2MgO + C ������ʽÿ����2�֣�
���������������1���������Ӧ����㷺�Ľ���������
��2���ؿ��к������Ľ���Ԫ��������
��3�����ƶ�G��ˮ������ˮ�����ڸ����·�����Ӧ����������������������
��4���������ȷ�Ӧ��
��5�����ƶ�L��һ����̼��Ȼ��K�Ƕ�����̼������ΪDԪ�غ�EԪ�������ڱ������ڣ�����E��þ��������̼��þ��ȼ��������þ��̼��
���㣺��ѧ��Ӧ����ʽ��

��ѧ��ѧ�кܶࡰ���ɡ����������÷�Χ,���и����йء����ɡ��Ƴ��Ľ��ۺ�������
| A��Na2O?Na2O2���Ԫ����ͬ,�Ƴ���ˮ��Ӧ����Ҳ��ȫ��ͬ |
| B��SO2��ʪ���Cl2����Ư����,�Ƴ�����Ϻ�Ư���Ը�ǿ |
| C��H2CO3�����Ա�HClOǿ,�Ƴ�CO2ͨ��NaClO��Һ��������HClO |
| D�����ݳ�����ͭ��Ũ���ᷴӦ������ȡNO2���Ƴ�����������Ũ���ᷴӦҲ������ȡNO2 |