��Ŀ����
ȡ��ͬ�����0.025 L��������0.01 mol��L-1��NaOH��Һ��������һ�ݷ��ڿ�����һ��ʱ�����Һ��pH_______���������С�����䡱������ԭ����__________________������֪Ũ�ȵ�������Һ�к�����������Һ�����к͵�һ�ݣ��ڿ����з���һ��ʱ��ģ������ĵ�������Һ�����ΪVA����һ��ΪVB����
(1)�Լ�����ָʾ��ʱ��VA��VB�Ĵ�С��ϵΪ__________________________________��
(2)�Է�̪��ָʾ��ʱ��VA��VB�Ĵ�С��ϵΪ____________________________________��
������NaOH��Һ¶���ڿ����л����տ����е�CO2������Na2CO3��ʹ��Һ�ļ��Լ�����pH��С����������ζ�ʱ����һ��NaOH��Һ�л���Na2CO3��Na2CO3��H2SO4�ķ�Ӧ���������У�![]() +H+====
+H+====![]() ���պ÷�Ӧʱ��Һ��pH�ӽ���8���Լ��ԣ���
���պ÷�Ӧʱ��Һ��pH�ӽ���8���Լ��ԣ���![]() +H+====H2O+CO2�����պ÷�Ӧʱ��Һ��pH�ӽ���4�������ԣ�����ѡ�ü�����ָʾ��ʱ����Һ�ı�ɫ��pHΪ4.4���ʷ���������Ӧ����ϵʽΪ2NaOH��Na2CO3��H2SO4���ɼ�����Ӱ������������������÷�̪��ָʾ������Һ�ı�ɫ��pHΪ8����������һ����Ӧ����ϵʽΪNaOH��NaHCO3��
+H+====H2O+CO2�����պ÷�Ӧʱ��Һ��pH�ӽ���4�������ԣ�����ѡ�ü�����ָʾ��ʱ����Һ�ı�ɫ��pHΪ4.4���ʷ���������Ӧ����ϵʽΪ2NaOH��Na2CO3��H2SO4���ɼ�����Ӱ������������������÷�̪��ָʾ������Һ�ı�ɫ��pHΪ8����������һ����Ӧ����ϵʽΪNaOH��NaHCO3��![]() H2SO4���ɼ�������������ƫС��
H2SO4���ɼ�������������ƫС��
�𰸣���С NaOH��Һ¶���ڿ����л����տ����е�CO2,����Na2CO3,ʹ��Һ�ļ��Լ�����pH��С (1)VA=VB (2)VA��VB

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�|
����ʱȡŨ����ͬ��NaOH��HCl��Һ����2��1��������ϣ�������Һ��pH����12����ԭ��Һ��Ũ��Ϊ | |
| [����] | |
A�� |
0.05 mol/L |
B�� |
0.03 mol/L |
C�� |
0.02 mol/L |
D�� |
0.50 mol/L |
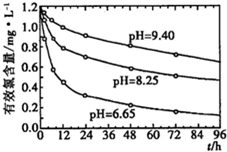
�������ҹ��Ѿ�������������ˮ��Ⱦ�¼�������������ˮ����ҵ��ˮ�Ĵ����ŷ�������Ҫԭ��ˮ�е� �Ǻ����л���ֽ�IJ����Ũ�ȵĴ�С��ˮ��Ⱦ�ı�־֮һ�����ˮ�е�
�Ǻ����л���ֽ�IJ����Ũ�ȵĴ�С��ˮ��Ⱦ�ı�־֮һ�����ˮ�е� ���ñ�ɫ�����䲽��Ϊ��
���ñ�ɫ�����䲽��Ϊ��
I����1�����Ʊ���Һ����ȡO��30g NaNO2���1000mL��ҺA���ò��������õ��IJ����������ձ�������ƿ���______��
��2����ȡ5��OO mL��ҺA��ϡ����1L������ҺB��ȷ��ȡ5.00mL��ҺAӦѡ�õļ���������______��
�����Ʊ�ɫ�ף�ȡ6ֻ����Ϊ10mL�ı�ɫ�ܣ��ֱ����������ȵ���ҺB������10mL���ټ�������Լ0.3g������������ �� ����ĩ��������£���
����ĩ��������£���
| ɫ����� | 1 | 2 | 3 | 4 | 5 | 6 |
| ������ҺB�������mL | 0.0 | 2.0 | 4.0 | 6.0 | 8.0 | 10.0 |
| ��Ӧ����Һ��ɫ | ����ɫ��Ϊ��dz�����ӣ�Һ�ɫ | |||||
��1����ɫ������______��ֵIJⶨ�������������������
��2����ˮ����ɫ����ɫ���е�6����ɫ��ͬ������ˮ���к�
 Ϊ______mg/L��������һλС������
Ϊ______mg/L��������һλС��������3����NaNO2����ֱ��������ҺB��ȱ����______��
��4�����ˮ����ɫ���6�Ż��Ӧ��ȡ�Ĵ�ʩ��______��
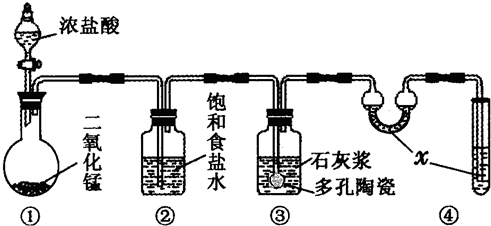
 ��ѧ���о����֣������������ڵ������ӣ��ɽ���1������ת��Ϊ�������������ɻ���O2������������������һϵ�����ɻ���һ������������������ھ���һ�������Ļ�����ϵͳ��������ø�Ϳ������������ܽ�������ת��Ϊ���Խϵ͵����ʣ���������ܵ����������������ǰ���NH2OH�������ķ������Լ��������ϵͳ��O2��������ԭ����O2�����ǰ���Ӧ����NO2����һ�ֹ������NO2���ڶ���������ͦ������������£����ɷۺ��ż��Ⱦ�壬Ⱦ���ڲ���530nm�����������գ���������ֵ��c(NO2��)�����ȣ��Ӷ��ɼ������Ʒ�е�O2��������ijʵ���������Ϸ�������������Һ��c(NO2��)��2.500��10-3 mol?L-1��
��ѧ���о����֣������������ڵ������ӣ��ɽ���1������ת��Ϊ�������������ɻ���O2������������������һϵ�����ɻ���һ������������������ھ���һ�������Ļ�����ϵͳ��������ø�Ϳ������������ܽ�������ת��Ϊ���Խϵ͵����ʣ���������ܵ����������������ǰ���NH2OH�������ķ������Լ��������ϵͳ��O2��������ԭ����O2�����ǰ���Ӧ����NO2����һ�ֹ������NO2���ڶ���������ͦ������������£����ɷۺ��ż��Ⱦ�壬Ⱦ���ڲ���530nm�����������գ���������ֵ��c(NO2��)�����ȣ��Ӷ��ɼ������Ʒ�е�O2��������ijʵ���������Ϸ�������������Һ��c(NO2��)��2.500��10-3 mol?L-1��