��Ŀ����
ij��������Ʒ�к���������FeSO4���ʡ�ijͬѧҪ�ⶨ������Ԫ�ص���������������������·������вⶨ����������Ϊ��

��������̻ش�
��1������I��������Һʱ�����õ��IJ����������ձ�����Ͳ������������ͷ�ι����⣬�������� (���������ƣ���
��2������II�б����õ��������� ��
��3����Ӧ���У���������H2O2��Һ��Ӧ�����ӷ���ʽ ��
��4�����������SO42���Ƿ����Ӹɾ��IJ���
��
��5������������ȣ���ȴ�����£�����ƽ������������Ⱥ�����������Ϊblg���ٴμ��Ȳ���ȴ�����³���������Ϊb2g����b1��b2=0.3����Ӧ���еIJ�����
��
��6��������������Ϊ42.6g��������������Ⱥ�ͬ���������Ϊ44.8g������Ʒ����Ԫ�ص���������= (����һλС������
��7����һͬѧ��Ϊ����������ʵ�鲽��̫����������Ϊ��ֻҪ����Ʒ����ˮ���ֽ��裬�����������ճ������ɣ���������������������Ƿ���У� ��������С������С���

��������̻ش�
��1������I��������Һʱ�����õ��IJ����������ձ�����Ͳ������������ͷ�ι����⣬�������� (���������ƣ���
��2������II�б����õ��������� ��
| A��50mL��Ͳ | B��100mL��Ͳ |
| C��50mL��ʽ�ζ��� | D��50mL��ʽ�ζ��� |
��4�����������SO42���Ƿ����Ӹɾ��IJ���
��
��5������������ȣ���ȴ�����£�����ƽ������������Ⱥ�����������Ϊblg���ٴμ��Ȳ���ȴ�����³���������Ϊb2g����b1��b2=0.3����Ӧ���еIJ�����
��
��6��������������Ϊ42.6g��������������Ⱥ�ͬ���������Ϊ44.8g������Ʒ����Ԫ�ص���������= (����һλС������
��7����һͬѧ��Ϊ����������ʵ�鲽��̫����������Ϊ��ֻҪ����Ʒ����ˮ���ֽ��裬�����������ճ������ɣ���������������������Ƿ���У� ��������С������С���
��16�֣���1��500mL����ƿ��2�֣� ��2��C��2�֣�����
��3��2Fe2++H2O2+2H+=2Fe3++2H2O��3�֣�
��4��ȡ�������һ��ϴ��Һ���Թ��У�����Ba(NO3)2����BaCl2����Һ�����ް�ɫ�����������ϴ����2�֣�
��5���������ȣ���ȴ�����¡�������ֱ�������������������0.1g��2�֣�
��6��55.0%��3�֣� ��7�������У�2�֣�
��3��2Fe2++H2O2+2H+=2Fe3++2H2O��3�֣�
��4��ȡ�������һ��ϴ��Һ���Թ��У�����Ba(NO3)2����BaCl2����Һ�����ް�ɫ�����������ϴ����2�֣�
��5���������ȣ���ȴ�����¡�������ֱ�������������������0.1g��2�֣�
��6��55.0%��3�֣� ��7�������У�2�֣�
�����������1����������һ�����ʵ���Ũ����Һ�ķ�����֪������500.00mL��Һ�Ⱥ���Ҫ�ձ�����Ͳ����������500mL����ƿ����ͷ�ιܵ���������2����Ͳ�ľ�ȷ��һ��Ϊ0.1mL���ζ��ܵľ�ȷ��һ��Ϊ0.01mL��Fe2O3+3H2SO4(����)=Fe2(SO4)3+3H2O������������������������Ļ����Һ�����ԣ���ȡ50.00mL������ҺӦ��ѡ��50mL��ʽ�ζ��ܣ���ֻ��C��ȷ����3��˫��ˮ�dz�����ɫ����������Ŀ���ǽ�������������Ϊ�����������ݻ��ϼ��������������������ԭ�Ӹ����غ��֪��2Fe2++H2O2+2H+=2Fe3++2H2O����4��H++NH3?H2O= H2O +NH4+��Fe3++3NH3?H2O=Fe(OH)3��+3NH4+��Fe(OH)3�������������ſ����Ե�����李���ˮ������SO42�D+Ba2+=BaSO4������˳��ÿ����Ա�����Һ����SO42�D�Ƿ�ϴ�Ӹɾ�����5��2Fe(OH)3
 Fe2O3+3H2O����b1��b2������ȣ�˵��Fe(OH)3����������ȫ�ֽ�ΪFe2O3���壬��b1��b2=0.3��˵��Fe(OH)3����û�г��ֽ⣬�����Ҫ��ʢ��Fe(OH)3�������������ж�μ��ȡ��ڸ���������ȴ����������¼������ֱ����������������ȣ���6��������������������m(Fe2O3)=44.8g��42.6g=2.2g��������Ԫ�ص�������Fe2O3=2Fe+3O����m(Fe)= m(Fe2O3)��112/160=��44.8g��42.6g����112/160��������Ԫ�������غ��֪��50.00mL����Һ��m(Fe)=��44.8g��42.6g����112/160����500.00mL����Һ��m(Fe)=��44.8g��42.6g����112/160��500.00mL/50.00mL��������Ԫ�������غ��֪��28.0g��Ʒ��m(Fe)=��44.8g��42.6g����112/160��500.00mL/50.00mL���������Ʒ����Ԫ�ص�����������w(Fe)=" m(Fe)/" m(��Ʒ)��100%=��44.8g��42.6g����112/160��500.00mL/50.00mL��28.0g��100%=55.0%����7����Ʒ��Fe2O3������ˮ��FeSO4������ˮ�������ܴٽ�FeSO4ˮ������Fe(OH)2��H2SO4��Fe(OH)2�ױ�����ΪFe(OH)3��H2SO4�ѻӷ���Fe(OH)3��H2SO4�����кͷ�Ӧ����Fe2(SO4)3��H2O���������ղ���ʹFe2(SO4)3��Һ�ֽ�ΪFe2O3���ʸ÷��������С�
Fe2O3+3H2O����b1��b2������ȣ�˵��Fe(OH)3����������ȫ�ֽ�ΪFe2O3���壬��b1��b2=0.3��˵��Fe(OH)3����û�г��ֽ⣬�����Ҫ��ʢ��Fe(OH)3�������������ж�μ��ȡ��ڸ���������ȴ����������¼������ֱ����������������ȣ���6��������������������m(Fe2O3)=44.8g��42.6g=2.2g��������Ԫ�ص�������Fe2O3=2Fe+3O����m(Fe)= m(Fe2O3)��112/160=��44.8g��42.6g����112/160��������Ԫ�������غ��֪��50.00mL����Һ��m(Fe)=��44.8g��42.6g����112/160����500.00mL����Һ��m(Fe)=��44.8g��42.6g����112/160��500.00mL/50.00mL��������Ԫ�������غ��֪��28.0g��Ʒ��m(Fe)=��44.8g��42.6g����112/160��500.00mL/50.00mL���������Ʒ����Ԫ�ص�����������w(Fe)=" m(Fe)/" m(��Ʒ)��100%=��44.8g��42.6g����112/160��500.00mL/50.00mL��28.0g��100%=55.0%����7����Ʒ��Fe2O3������ˮ��FeSO4������ˮ�������ܴٽ�FeSO4ˮ������Fe(OH)2��H2SO4��Fe(OH)2�ױ�����ΪFe(OH)3��H2SO4�ѻӷ���Fe(OH)3��H2SO4�����кͷ�Ӧ����Fe2(SO4)3��H2O���������ղ���ʹFe2(SO4)3��Һ�ֽ�ΪFe2O3���ʸ÷��������С�
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
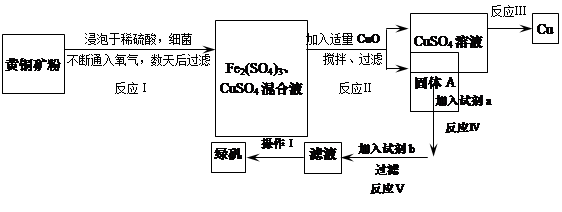
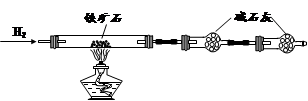
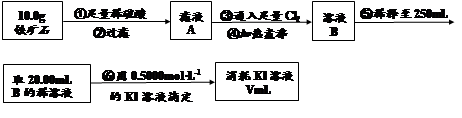
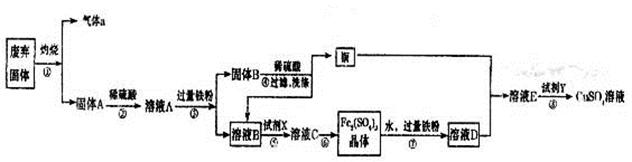
 ����
���� ˮ��������
ˮ�������� С�⣬����___________________________________��
С�⣬����___________________________________��