��Ŀ����
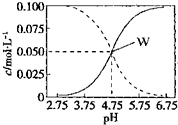 ��2013?����ģ�⣩25��ʱ��c��CH3COOH��+c��CH3COO-��=0.1mol?L-1�Ĵ��ᡢ�����ƻ����Һ�У�c��CH3COOH����c��CH3COO-����pH�Ĺ�ϵ��ͼ��ʾ�������йظ���Һ����������ȷ���ǣ�������
��2013?����ģ�⣩25��ʱ��c��CH3COOH��+c��CH3COO-��=0.1mol?L-1�Ĵ��ᡢ�����ƻ����Һ�У�c��CH3COOH����c��CH3COO-����pH�Ĺ�ϵ��ͼ��ʾ�������йظ���Һ����������ȷ���ǣ�������������A����ͼ��֪��pH=4.75ʱ��c��CH3COOH��=c��CH3COO-��=0.05mol/L��pH=4ʱ��������ǿ����Һ�д���ĵ���̶ȼ�С������Һ��c��CH3COOH��������Һ��c��CH3COO-����С���ݴ�ȷ��c��CH3COOH����c��CH3COO-������Դ�С��
B����ͼ��֪��W������ʾ����Һ��c��CH3COOH��=c��CH3COO-��=0.05mol/L�������Һ�е���غ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-���жϣ�
C��W������ʾ���Ǵ���ʹ����ƻ����Һ����Һϡ��10�������ڴ�����ڵ���ƽ�⣬�ٽ�������룬pH����С��1��
D����Һ��c��CH3COOH��+c��CH3COO-��=0.1mol?L-1�����ݵ���غ���c��Na+��+c��H+��=c��CH3COO-��+c��OH-�������������غ��� c��CH3COOH��+c��CH3COO-��=0.1 mol?L-1���ɵ���غ���c��Na+��+c��H+��=c��CH3COO-��+c��OH-��+c��Cl-�����ݴ˽��
B����ͼ��֪��W������ʾ����Һ��c��CH3COOH��=c��CH3COO-��=0.05mol/L�������Һ�е���غ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-���жϣ�
C��W������ʾ���Ǵ���ʹ����ƻ����Һ����Һϡ��10�������ڴ�����ڵ���ƽ�⣬�ٽ�������룬pH����С��1��
D����Һ��c��CH3COOH��+c��CH3COO-��=0.1mol?L-1�����ݵ���غ���c��Na+��+c��H+��=c��CH3COO-��+c��OH-�������������غ��� c��CH3COOH��+c��CH3COO-��=0.1 mol?L-1���ɵ���غ���c��Na+��+c��H+��=c��CH3COO-��+c��OH-��+c��Cl-�����ݴ˽��
����⣺A�� pH=4����Һ�У�c��H+����c��OH-�������ݵ���غ�c��CH3COO-����c��Na+����pH=4.75ʱ��c��CH3COOH��=c��CH3COO-��=0.05mol/L��pH=4ʱ��������ǿ������c��CH3COO-����c��CH3COOH������A��ȷ��
B����ͼ��֪��W������ʾ����Һ��c��CH3COOH��=c��CH3COO-��=0.05mol/L����Һ�е���غ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-��������c��Na+��+c��H+��=c��CH3COOH��+c��OH-������B��ȷ��
C��W������ʾ���Ǵ���ʹ����ƻ����Һ����Һϡ��10�������ڴ�����ڵ���ƽ�⣬�ٽ�������룬PH����С��1����W������ʾ��1.0L��Һϡ�͵�10L����pH�Ʋ�������Һ��pHӦС��5.75����C����
D����Һ��c��CH3COOH��+c��CH3COO-��=0.1mol?L-1������غ���c��Na+��+c��H+��=c��CH3COO-��+c��OH-��������c��Na+��+c��H+��-c��OH-��+c��CH3COOH��=0.1mol?L-1����D��ȷ��
��ѡ��C��
B����ͼ��֪��W������ʾ����Һ��c��CH3COOH��=c��CH3COO-��=0.05mol/L����Һ�е���غ�c��Na+��+c��H+��=c��CH3COO-��+c��OH-��������c��Na+��+c��H+��=c��CH3COOH��+c��OH-������B��ȷ��
C��W������ʾ���Ǵ���ʹ����ƻ����Һ����Һϡ��10�������ڴ�����ڵ���ƽ�⣬�ٽ�������룬PH����С��1����W������ʾ��1.0L��Һϡ�͵�10L����pH�Ʋ�������Һ��pHӦС��5.75����C����
D����Һ��c��CH3COOH��+c��CH3COO-��=0.1mol?L-1������غ���c��Na+��+c��H+��=c��CH3COO-��+c��OH-��������c��Na+��+c��H+��-c��OH-��+c��CH3COOH��=0.1mol?L-1����D��ȷ��
��ѡ��C��
���������⿼������Ũ�ȴ�С�ıȽϣ���Һ�������غ㡢����غ㣬��Һϡ�͡�������ʵ���ƽ�⣬��ȷ����ˮ�⡢�����غ��ǽ����Ĺؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ



