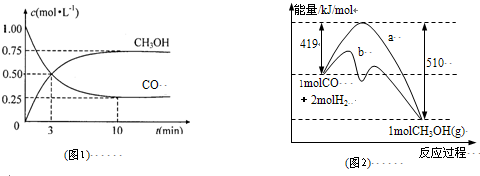
��Ŀ����
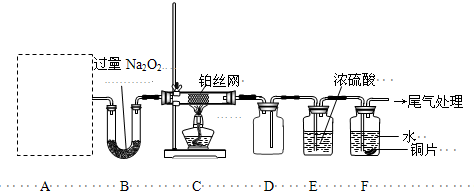
�����������ȵ�2.4gľ̿Ͷ��ʢ������12mol��L-1��ŨHNO3���Թ��У������������ӣ���ͼ��ʾ�����Ӻã������Ѽ��װ�õ������ԣ�����ش�

��1����Ӧ��ʼ��ȥ�ƾ��ƣ��Թ�a�г�����ľ̿����ȼ�գ������ʧ�⣬���۲쵽��������_______________________________��
��2���Թ���b����______������������С������ޡ�����ԭ����_________________________��
��3���Թ�c�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________��
��4������ƿe������ռ���������Ϊ______________________�������ϸ�����������Ϊ_________L����״��ʱ����ʵ���������ֵ________________�����С����
��2���Թ���b����______������������С������ޡ�����ԭ����_________________________��
��3���Թ�c�з�����Ӧ�Ļ�ѧ����ʽΪ_________________________________��
��4������ƿe������ռ���������Ϊ______________________�������ϸ�����������Ϊ_________L����״��ʱ����ʵ���������ֵ________________�����С����
��1���Թ��ϲ���������ɫ����
��2���ޣ�NO2��ˮ��Ӧ������������HNO3���к���Ca(OH)2
��3��Ba(OH)2+CO2=BaCO3��+H2O��Ba(OH)2+CO2+H2O=Ba(HCO3)2
��4��һ��������6.0��С
��2���ޣ�NO2��ˮ��Ӧ������������HNO3���к���Ca(OH)2
��3��Ba(OH)2+CO2=BaCO3��+H2O��Ba(OH)2+CO2+H2O=Ba(HCO3)2
��4��һ��������6.0��С

��ϰ��ϵ�д�
 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д�
�����Ŀ