��Ŀ����
�̶�������CO2����Ч��������Դ�������ٿ����е��������塣��ҵ����һ����CO2�������״�ȼ�ϵķ�����CO2(g)��3H2(g) CH3OH(g)��H2O(g) ��H����49��0 kJ��mol-1ij��ѧʵ�齫6molCO2��8molH2����
CH3OH(g)��H2O(g) ��H����49��0 kJ��mol-1ij��ѧʵ�齫6molCO2��8molH2����
2L���ܱ������У����H2�����ʵ�����ʱ��仯����ͼ��ʾ��ʵ�ߣ���
 CH3OH(g)��H2O(g) ��H����49��0 kJ��mol-1ij��ѧʵ�齫6molCO2��8molH2����
CH3OH(g)��H2O(g) ��H����49��0 kJ��mol-1ij��ѧʵ�齫6molCO2��8molH2����2L���ܱ������У����H2�����ʵ�����ʱ��仯����ͼ��ʾ��ʵ�ߣ���

��1��a������Ӧ����_______������ڡ����ڻ�С�ڣ��淴Ӧ���ʡ�
��2������ʱ���ƽ����Ӧ����������__________����С����__________��
A��0��1min B��1��3min C��3��8min D��8��11min
��3����ƽ��ʱ������ת���ʺ������·�Ӧ��ƽ�ⳣ��K����д��������̣�
_____________________________________
��4�����ı�ijһʵ�������ٽ�������ʵ�飬���H2�����ʵ�����ʱ��仯��ͼ��������ʾ������I��Ӧ��ʵ�������ı���___________������II��Ӧ��ʵ�������ı���___________��
��2������ʱ���ƽ����Ӧ����������__________����С����__________��
A��0��1min B��1��3min C��3��8min D��8��11min
��3����ƽ��ʱ������ת���ʺ������·�Ӧ��ƽ�ⳣ��K����д��������̣�
_____________________________________
��4�����ı�ijһʵ�������ٽ�������ʵ�飬���H2�����ʵ�����ʱ��仯��ͼ��������ʾ������I��Ӧ��ʵ�������ı���___________������II��Ӧ��ʵ�������ı���___________��
��1������
��2��A��D
��3���⣺��������CO2��g����3H2��g�� CH3OH��g����H2O��g��
CH3OH��g����H2O��g��
��ʼ���ʵ���/mol������6������8��������������0����������0
���ʵ����仯/mol������2������6��������������2����������2
ƽ�����ʵ���/mol������4������2��������������2����������2
H2��ת���ʣ� ��100����
��100���� ��75��
��75��

��4�������¶ȣ�����ѹǿ
��2��A��D
��3���⣺��������CO2��g����3H2��g��
 CH3OH��g����H2O��g��
CH3OH��g����H2O��g�� ��ʼ���ʵ���/mol������6������8��������������0����������0
���ʵ����仯/mol������2������6��������������2����������2
ƽ�����ʵ���/mol������4������2��������������2����������2
H2��ת���ʣ�
 ��100����
��100���� ��75��
��75��
��4�������¶ȣ�����ѹǿ

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ

 ͨ����ȥ��Ӧ�Ʊ���Ļ�ѧ����ʽΪ
ͨ����ȥ��Ӧ�Ʊ���Ļ�ѧ����ʽΪ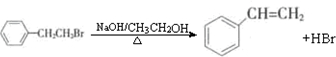

 ����дһ�֣�
����дһ�֣� ��H2���߷�����Ӧ�����������뷴Ӧ�������ɻ�������͢��������ʽ��ΪC9H8O���Ҷ��ܷ���������Ӧ�����й��ڢ��͢���˵����ȷ����
��H2���߷�����Ӧ�����������뷴Ӧ�������ɻ�������͢��������ʽ��ΪC9H8O���Ҷ��ܷ���������Ӧ�����й��ڢ��͢���˵����ȷ����
 �̶�������CO2����Ч��������Դ�������ٿ����е��������壮��ҵ����һ����CO2�������״�ȼ�ϵķ�����
�̶�������CO2����Ч��������Դ�������ٿ����е��������壮��ҵ����һ����CO2�������״�ȼ�ϵķ����� CH3OH��g��+H2O��g��+49kJ
CH3OH��g��+H2O��g��+49kJ
 CH3OH(g)��H2O(g) ��H ��
��49.0 kJ��mol��1��
CH3OH(g)��H2O(g) ��H ��
��49.0 kJ��mol��1��