��Ŀ����
����A��B��C��D��E�����л�����ǵ���ɶ���̼40%����6.7%����53.3%����֪��1��A��H2������ܶ�Ϊ15����ˮ��Һ�ܷ���������Ӧ����2��B�����й���24��ԭ�ӣ�B��C��Ϊͬ���칹�壬B��C��������ζ�İ�ɫ���壬��C��B�𣻣�3��D��E�������ܶ�Ϊ2.68 g��L��1����״������D�������ԣ�E��ˮ��õ������ᡢ������������д��A��B��C��D��E�����ƺͽṹ��ʽ��
������
A����ȩHCHO B��������C6H12O6 C������C6H12O6 D������CH3COOH E���������
|
��ʾ��
A��H2�ܶ�15 ��ΪH2ʽ��Ϊ2 ����Aʽ��Ϊ15��2��30 ��ΪA��C��H��O�����ֱ�Ϊ40%��6.7%��53.3% ����A��C���� ��A����ʽCH2O ��A�ܷ���������Ӧ ����A����ȩ�� ����AΪ��ȩ�� �����⣬���뵽B��C�������࣬C��B����B��C����24��ԭ�� ����BΪ������ CΪ���� E��D�����ܶ�Ϊ2.68 g��L��1����״��������ΪĦ��������Ħ��������ܶȣ�����E��DĦ������Ϊ��2.68 g��L��1��22.4 L��mol��1��60 g��mol��1������C��H��O�ٷֺ����������D��E����ʽΪC2H4O2 ��ΪD�����ԣ�DΪ����CH3COOH��E��ˮ��������ʹ���EΪ���࣬�ṹʽΪ
|

 �����������
��Ϊ���������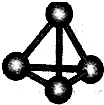 ����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��
����A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������D��E���⻯����ӹ��Ͷ���V�ͣ�A��B������������֮����C��������������ȣ�A�ֱܷ���B��C��D�γɵ���������ȵķ��ӣ���A��D���γɵĻ���������¾�ΪҺ̬��






