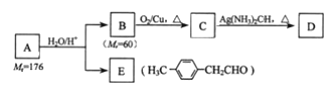
��Ŀ����
����Ŀ��п��ԭ���Ȼ�����һ����������Ӧ��ǰ�����Ʊ���ķ��������Ʊ�����ʾ��ͼ��ͼ��

(1)1 mol��̿�ڹ�������ʧȥ___mol���ӡ�
(2)��������Cl2�õ�ⱥ��ʳ��ˮ�Ʊ����Ʊ�Cl2�����ӷ���ʽΪ___��
(3)�����������̱����ϸ������ˮ���ش��������⣺
��SiCl4��ˮ����ˮ������SiO2��һ���ᣬ��ѧ��Ӧ����ʽΪ_______��
������Cl2ʱ�������ڳ�ָ���Ͳ�����ȫ�ĽǶȿ��ǣ��轫Լ90���ij�ʪ��������ȴ��12����Ȼ����ͨ��ŨH2SO4�С���ȴ��������___��
(4)Zn��ԭSiCl4�Ŀ��淴Ӧ���ң�SiCl4(g)+2Zn(s)![]() Si(s)+2ZnCl2(g) ��H<0����˵����ȷ������__________��
Si(s)+2ZnCl2(g) ��H<0����˵����ȷ������__________��
A����ԭ�������������������н���
B��Ͷ��1 mol Zn������14 g��ԭ����
C������SiCl4(g)�������÷�Ӧƽ�ⳣ������
D����SiCl4(g)������������H��ֵ
(5)��һ���ɹ������IJ����г�����Ag2SO4����֪ij�¶���Ag2SO4(M=312 g/mol)���ܽ��Ϊ0.624 g/100 gH2O(����Һ���Ϊˮ�����)�����¶���Ag2SO4��Ksp=___����λ��Ч���֣���
���𰸡�2 2Cl-+2H2O![]() Cl2��+H2��+2OH- SiCl4+2H2O=SiO2+4HCl ʹˮ�������������ٽ���Ũ�����ˮ������Ũ�����������ˮ�ԣ�ͬʱ���ͷų������� AC 3.2��10-5
Cl2��+H2��+2OH- SiCl4+2H2O=SiO2+4HCl ʹˮ�������������ٽ���Ũ�����ˮ������Ũ�����������ˮ�ԣ�ͬʱ���ͷų������� AC 3.2��10-5
��������
ʯӢɰ�뽹̿�ڸ����·�Ӧ�����ֹ裬�õ��Ĵֹ����ⷽ��������������Ӧ����SiCl4��SiCl4�ڸ����±�Zn��ԭ������Si������SiCl4��ˮ����ˮ�ⷴӦ�����Է�Ӧ����Ҫ�ϸ������ˮ�����ڿ��淴Ӧ����Ϸ�Ӧ�ص㼰��ѧƽ���ƶ�ԭ�������жϣ����������Ե����ʣ��ɽ���ܽ�����ܶȻ������ĺ��壬�����ʵ��ܽ�ȼ�������Ũ�ȣ����õ����ʵ��ܶȻ�����Ksp��ֵ��
(1)����I�з�����Ӧ��SiO2+2C![]() Si+2CO�����ڷ�Ӧ�У�̼Ԫ�ػ��ϼ���0������Ϊ+2�ۣ�����1 mol��̿�ڹ��̢���ʧȥ2 mol���ӣ�
Si+2CO�����ڷ�Ӧ�У�̼Ԫ�ػ��ϼ���0������Ϊ+2�ۣ�����1 mol��̿�ڹ��̢���ʧȥ2 mol���ӣ�
(2)����Ȼ�����Һ�����������ơ�������������������ӷ���ʽΪ��2Cl-+2H2O![]() Cl2��+H2��+2OH-��
Cl2��+H2��+2OH-��
(3)��SiCl4��ˮ����ˮ������SiO2��һ���ᣬ����Ԫ���غ㣬��֪����Ϊ���ᣬ��Ӧ�Ļ�ѧ����ʽΪ��SiCl4+2H2O=SiO2+4HCl��
�ڸ���Cl2ʱ�������ڳ�ָ���Ͳ�����ȫ�ĽǶȿ��ǣ��ɽ�Լ90��ij�ʪ��������ȴ��12�棬Ȼ����ͨ��ŨH2SO4�У���ʹˮ�������������ٽ���Ũ�����ˮ������Ũ�����������ˮ�ԣ�ͬʱ���ͷų���������
(4)��ӦSiCl4(g)+2Zn(s)![]() Si(S)+2ZnCl2(g) ��H<0������ӦΪ�����������ķ��ȷ�Ӧ��
Si(S)+2ZnCl2(g) ��H<0������ӦΪ�����������ķ��ȷ�Ӧ��
A��Zn��Si�ڸ��������¶�����������Ӧ�����Ըù������������������н��У�A��ȷ��
B����Ӧ���ڿ��淴Ӧ��Ͷ���Zn������ȫ��Ӧ��Ͷ��1 mol Zn��Ӧ���ɻ�ԭ����Si�����ʵ���С��0.5 mol����������С��14 g��B����
C��ƽ�ⳣ��ֻ���¶��йأ�����SiCl4(g)��������Ӧ�¶Ȳ��䣬��÷�Ӧƽ�ⳣ�����䣬C��ȷ��
D���ʱ���H���Ȼ�ѧ����ʽ��SiCl4�����ʵ����йأ�������SiCl4�����أ�D����
�ʺ���ѡ����AC��
(5)����ˮ������Ϊ100 g����100 gˮ�ܽ��Ag2SO4������Ϊ0.624 g����n(Ag2SO4)=![]() =2��10-3 mol����Һ������Լ100.624 g����Һ�ܶ�Լ��ˮ���ܶȣ�������Һ���ԼΪ100.624 mL��0.1 L������Ag2SO4�����ʵ���Ũ��c(Ag2SO4)=2��10-2 mol/L����c(SO42-)=2��10-2 mol/L��c(Ag+)=2c(SO42-)=4��10-2 mol/L������Ksp(Ag2SO4)= c2(Ag+) c(SO42-)=(4��10-2)2��2��10-2=3.2��10-5��
=2��10-3 mol����Һ������Լ100.624 g����Һ�ܶ�Լ��ˮ���ܶȣ�������Һ���ԼΪ100.624 mL��0.1 L������Ag2SO4�����ʵ���Ũ��c(Ag2SO4)=2��10-2 mol/L����c(SO42-)=2��10-2 mol/L��c(Ag+)=2c(SO42-)=4��10-2 mol/L������Ksp(Ag2SO4)= c2(Ag+) c(SO42-)=(4��10-2)2��2��10-2=3.2��10-5��

 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�����Ŀ������ͼװ���л�����ͨ������X�����رջ�������Ʒ����Һ�ޱ仯��������ʯ��ˮ����ǣ�����������Ʒ����Һ��ɫ�����Ⱥ��ָֻ���ɫ���ݴ��ж�����X��ϴ��ƿ����ҺY�ֱ������ ��������
A | B | C | D | |
X | SO2 | H2S | CO2 | Cl2 |
Y | ����NaHCO3 | Ũ���� | Na2SO3 | NaHCO3 |

A.A��ȷB.B��ȷC.C��ȷD.D��ȷ
����Ŀ��[2016���¿α�I]��(Ge)�ǵ��͵İ뵼��Ԫ�أ��ڵ��ӡ����ϵ�����Ӧ�ù㷺���ش��������⣺
��1����̬Geԭ�ӵĺ�������Ų�ʽΪ[Ar]_______________����__________��δ�ɶԵ��ӡ�
��2��Ge��C��ͬ��Ԫ�أ�Cԭ��֮������γ�˫������������Geԭ��֮�������γ�˫������������ԭ�ӽṹ�Ƕȷ�����ԭ����______________________________��
��3���Ƚ�������±������۵�ͷе㣬������仯���ɼ�ԭ��______________________________��
GeCl4 | GeBr4 | GeI4 | |
�۵�/�� | 49.5 | 26 | 146 |
�е�/�� | 83.1 | 186 | Լ400 |
��4�������ԭCO2�Ʊ�CH4��Ӧ�У���״����Zn2GeO4�Ǹ÷�Ӧ�����ô�����Zn��Ge��O�縺���ɴ���С��˳����______________________________��
��5��Ge�������н��ʯ�ͽṹ������Geԭ�ӵ��ӻ���ʽΪ_______________����֮����ڵ���������_______________��
��6����������������Ҫ�أ�
��ԭ�������������ʾ�����ڲ���ԭ�ӵ����λ�á���ͼΪGe�����ľ���������ԭ���������AΪ(0��0��0)��BΪ(![]() ��0��
��0��![]() )��CΪ(
)��CΪ(![]() ��
��![]() ��0)����Dԭ�ӵ��������Ϊ_______________��
��0)����Dԭ�ӵ��������Ϊ_______________��

���������������������Ĵ�С����״����֪Ge�����ľ�������a=565.76 pm�����ܶ�Ϊ_____g��cm3(�г�����ʽ����)��