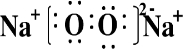��Ŀ����
Ԫ��X��Y��Z��W��Ϊ������Ԫ�أ���ԭ����������������֪Yԭ������������ռ�������������3/4��W����Z����X���뾶��С��������XW������Ϊ���壬Z�DZ������г�ϡ������Ԫ���⣬ԭ�Ӱ뾶����Ԫ�أ��ݴ˻ش��������⣺
(1)W��Ԫ�����ڱ��е�λ�ã�________����ҵ������W���ʵ����ӷ���ʽΪ��_____________________________________��
(2)A��B��Ϊ����������Ԫ���е�������ɵ�ǿ����ʣ��ҳ������������ʵ�ˮ��ҺpH������7�����Ԫ�ص�ԭ����Ŀ�Ⱦ�Ϊ1��1��1����A������ˮ�ĵ��룬��B�ܴٽ�ˮ�ĵ��룬��A��B�Ļ�ѧʽ�ֱ�Ϊ________��________��
(3)C������������Ԫ�ص�������ɵ�һ�ֺ��зǼ��Լ������ӻ������C�ĵ���ʽΪ __________________________��
(1)W��Ԫ�����ڱ��е�λ�ã�________����ҵ������W���ʵ����ӷ���ʽΪ��_____________________________________��
(2)A��B��Ϊ����������Ԫ���е�������ɵ�ǿ����ʣ��ҳ������������ʵ�ˮ��ҺpH������7�����Ԫ�ص�ԭ����Ŀ�Ⱦ�Ϊ1��1��1����A������ˮ�ĵ��룬��B�ܴٽ�ˮ�ĵ��룬��A��B�Ļ�ѧʽ�ֱ�Ϊ________��________��
(3)C������������Ԫ�ص�������ɵ�һ�ֺ��зǼ��Լ������ӻ������C�ĵ���ʽΪ __________________________��
(1)�������ڵڢ�A�壻 2Cl����2H2O H2����Cl2����2OH��
H2����Cl2����2OH��
(2)NaOH��NaClO
(3)
 H2����Cl2����2OH��
H2����Cl2����2OH�� (2)NaOH��NaClO
(3)


��ϰ��ϵ�д�
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
�����Ŀ