��Ŀ����
�������ƣ�NaN3���ǿ�������ҩ���ơ��ϳɹ����е��м�������ʡ�
��1��NaN3�д��ڵĻ�ѧ����________________���𰸿��ܲ�ֹһ��������ĸ����
a�����Ӽ� b�����Թ��ۼ� c���Ǽ��Թ��ۼ�
��2��3mol NaN3��ײ��������4mol ������һ�����ӻ�����A�������������������ҡ���д���йط�Ӧ�Ļ�ѧ����ʽ______________________��������������
��֪X��Y��Z��W�Ƕ������е����ַǽ���Ԫ�أ����ǵ�ԭ��������������XԪ��ԭ���γɵ����Ӿ���һ�����ӣ�Z��W��Ԫ�����ڱ��д������ڵ�λ�ã����ǵĵ����ڳ����¾�Ϊ��ɫ���壬Yԭ�ӵ��������������ڲ��������2����
��3������X��Z��W����Ԫ����ɵ�ij������һ����Ч���ϣ�������ʩ�û�ʹ�����ữ���йص����ӷ���ʽΪ______________________��
��4��������Ԫ�ؿ����ԭ�Ӹ�����Ϊ5:1:1:3�Ļ������X��Y��Z��W��˳���û������ˮ��Һ��������NaOH��Һ��Ӧ�����ӷ���ʽΪ��______________________��
��1��NaN3�д��ڵĻ�ѧ����________________���𰸿��ܲ�ֹһ��������ĸ����
a�����Ӽ� b�����Թ��ۼ� c���Ǽ��Թ��ۼ�
��2��3mol NaN3��ײ��������4mol ������һ�����ӻ�����A�������������������ҡ���д���йط�Ӧ�Ļ�ѧ����ʽ______________________��������������
��֪X��Y��Z��W�Ƕ������е����ַǽ���Ԫ�أ����ǵ�ԭ��������������XԪ��ԭ���γɵ����Ӿ���һ�����ӣ�Z��W��Ԫ�����ڱ��д������ڵ�λ�ã����ǵĵ����ڳ����¾�Ϊ��ɫ���壬Yԭ�ӵ��������������ڲ��������2����
��3������X��Z��W����Ԫ����ɵ�ij������һ����Ч���ϣ�������ʩ�û�ʹ�����ữ���йص����ӷ���ʽΪ______________________��
��4��������Ԫ�ؿ����ԭ�Ӹ�����Ϊ5:1:1:3�Ļ������X��Y��Z��W��˳���û������ˮ��Һ��������NaOH��Һ��Ӧ�����ӷ���ʽΪ��______________________��
��1��ac
��2��3NaN3==Na3N+4N2��
��3��NH4++H2O==NH3��H2O+H+
��4��NH4++HCO3-+2OH-==NH3��+2H2O+CO32-
��2��3NaN3==Na3N+4N2��
��3��NH4++H2O==NH3��H2O+H+
��4��NH4++HCO3-+2OH-==NH3��+2H2O+CO32-

��ϰ��ϵ�д�
�����Ŀ
�������ƣ�NaN3������ɫ���Ӿ��壬�����»�������ܷ���ǿ�ұ�ը�����ٷֽ���Ƶ��ʺ͵�������������ȫ�����еijɷ�֮һ�������й�˵����ȷ���ǣ�������
| A��NaN3��ֻ�������Ӽ� | B��1molNaN3��ȫ�ֽ������Բ���33.6LN2 | C��NaN3���ٷֽ�ʱ��NaN3���������������ǻ�ԭ�� | D�������£�NaN3��ѧ�����ȶ�����װ���������ر�˵��ע������ |
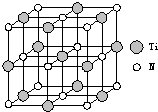 ��2013?������ģ����5-��������
��2013?������ģ����5-��������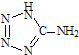 ���м������Ga���õ�������һ���������巢������������������ȫ���ң�
���м������Ga���õ�������һ���������巢������������������ȫ���ң�