��Ŀ����
��15�֣����������з�Ӧ�ϳ������谷��CaO��3CCaC2��CO����CaC2��N2CaCN2��C��CaCN2��2H2O===NH2CN��Ca(OH)2��NH2CN��ˮ��Ӧ��������[CO(NH2)2]�����غϳ������谷��
(1)д����Ca��ͬһ������������������ͬ���ڲ��������ӵĻ�̬ԭ�ӵĵ����Ų�ʽ��______________________________________________________________________��
CaCN2��������ΪCN����CN��Ϊ�ȵ�����ķ�����N2O��________(�ѧʽ)���ɴ˿�����֪CN�Ŀռ乹��Ϊ________��
(2)���ط�����Cԭ�Ӳ�ȡ________�ӻ������ط��ӵĽṹ��ʽ��________________��
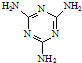
(3)�����谷  �׳ơ����������������������谷����������
�׳ơ����������������������谷���������� ��
��
���������������谷�����֮��ͨ��________��ϣ������������γɽ�ʯ��
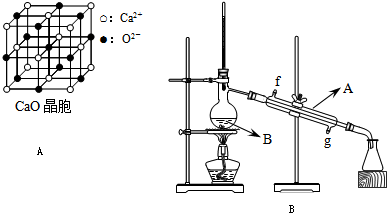
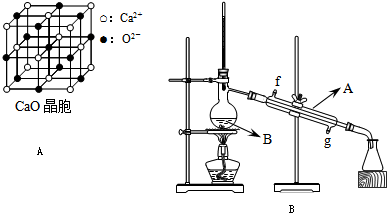
(4)CaO������ͼ��ʾ��CaO������Ca2+����λ��Ϊ______________��

��֪CaO������ܶ�Ϊ�ѣ����о������������������֮��ľ��� ________________ __���г�����ʽ��
CaO�����NaCl����ľ����ֱܷ�Ϊ��CaO 3 401 kJ��mol-1��NaCl 786 kJ��mol-1���������߾����ܲ������Ҫԭ����______________ ______��
(1)1s22s22p63s23p63d104s2��[Ar]3d104s2��(2��) CO2��1�֣� ֱ���� ��1�֣�
(2)sp2����1�֣�COH2NNH2 ��2�֣� (3)���Ӽ���� ��1�֣�(4)6����2�֣� ��3�֣�
��3�֣�
CaO������Ca2����O2���Ĵ���������NaCl������Na����Cl���Ĵ�������2�֣�
��������
���������
(1) Ca�ڵ������ڣ��������IJ���ӣ������2�����ӣ����ڲ��������ӵĻ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d104s2��[Ar]3d104s2��CN��16�����ӣ���CN��Ϊ�ȵ�����ķ��Ӻ���CO2���ɴ˿�����֪CN�Ŀռ乹����CO2����Ϊֱ���Σ�
(2) ���ط�����Cԭ������ԭ�ӽ�ϳ�˫������������ԭ�ӽ�ϳɵ�������ȡsp2�ӻ������ط��ӵĽṹ��ʽΪCOH2NNH2��
(3) ���谷�����к���NԪ�أ��֮���ͨ�����Ӽ������ϣ�
(4) ��ͼ��֪��CaO������Ca2+����λ��Ϊ6�� CaO�����NaCl���嶼�����Ӿ��壬�����ܲ������Ҫԭ����CaO������Ca2����O2���Ĵ���������NaCl������Na����Cl���Ĵ�������
���㣺���ʵĽṹ
���������⿼�����ʵĽṹ���漰�����ӽṹ���ӻ�����ȳ������ݣ�����һ���Ѷȡ���������Ŀ�������ڸ߿����ѱ����������������ͣ�Ҫ�������зḻ����������


 ���׳ơ����������������������谷���������ᣨ
���׳ơ����������������������谷���������ᣨ 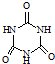 �������������������谷�����֮��ͨ��
�������������������谷�����֮��ͨ�� �׳ơ����������������������谷����������
�׳ơ����������������������谷���������� ��
��
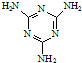
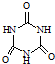
 )�׳ơ����������������������谷����������(
)�׳ơ����������������������谷����������( )�����������������谷�����֮��ͨ��________��ϣ������������γɽ�ʯ��
)�����������������谷�����֮��ͨ��________��ϣ������������γɽ�ʯ�� (4)CaO������ͼ��ʾ��CaO������Ca2+����λ��Ϊ____________________________________________________��
(4)CaO������ͼ��ʾ��CaO������Ca2+����λ��Ϊ____________________________________________________��