��Ŀ����
��
25�桢101 kPaʱ��1 molijҺ̬����CnH2n+2(l)��ȫȼ�����ɶ�����̼�����Һ̬ˮʱ���ų�����5518 kJ�����������������Ӧ�ķ�Ӧ�ȣ���
nC(s)��(n��1)H2(g)��CnH2n+2(l)����H1����202.2 kJ/mol��
C(s)��O2(g)��
H2(g)����д��������ȼ�յ��Ȼ�ѧ����ʽ��
�𰸣�

��ϰ��ϵ�д�
�����Ŀ
��10�֣�����̼ѭ�����Ѿ������˹�������ӣ��Իش��������⣺
��1��ú��������Һ���������ȼ�ϵ������ʡ�
��֪25�棬101 ʱ��
ʱ��

����25�棬101 ʱ��
ʱ�� .
.
��2����¯������CO�������Ҫ��;֮һ���������ӦΪ��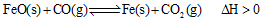 ����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
���¶����ߣ���ѧƽ���ƶ���ﵽ�µ� ƽ�⣬��ʱƽ�ⳣ��Kֵ �����������С�����䡱����
ƽ�⣬��ʱƽ�ⳣ��Kֵ �����������С�����䡱����
��1100��ʱ��ø�¯ʱ�� ������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬�� (��ǡ���)�����ж������� ��
������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬�� (��ǡ���)�����ж������� ��
��Ŀǰ��ҵ�Ͽ��� ������ȼ�ϼ״�
������ȼ�ϼ״� ���йط�ӦΪ��
���йط�ӦΪ�� ���������Ϊ1L���ܱ������У�����1mol
���������Ϊ1L���ܱ������У�����1mol ��3mol
��3mol ����Ӧ�����в��
����Ӧ�����в�� ��
�� (g)��Ũ����ʱ��ı仯��ͼ��ʾ��
(g)��Ũ����ʱ��ı仯��ͼ��ʾ��
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ���� ��
��
�����д�ʩ��ʹ �������
�������  ������ţ���
������ţ���
| A�������¶� |
B���ٳ��� |
C���ٳ��� |
D���� ��g������ϵ�з��� ��g������ϵ�з��� |
 ��
�� Fe(s)��CO2(g) ��H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
Fe(s)��CO2(g) ��H>0����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
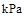 ʱ��
ʱ��

 .
. ����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263��
����֪��1100��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ��K=0.263�� ������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬�� (��ǡ���)�����ж�������
��
������������£��÷�Ӧ�Ƿ��ڻ�ѧƽ��״̬�� (��ǡ���)�����ж�������
�� ������ȼ�ϼ״����йط�ӦΪ��
������ȼ�ϼ״����йط�ӦΪ�� ���������Ϊ1L���ܱ������У�����1mol
���������Ϊ1L���ܱ������У�����1mol ����Ӧ�����в��
����Ӧ�����в�� (g)��Ũ����ʱ��ı仯��ͼ��ʾ��
(g)��Ũ����ʱ��ı仯��ͼ��ʾ��
 ��
�� ������� ������ţ���
������� ������ţ��� ��g������ϵ�з���
E.����He(g),ʹ��ϵѹǿ����
��g������ϵ�з���
E.����He(g),ʹ��ϵѹǿ����