��Ŀ����
����Ŀ���������������Ҫ��ҽ���м��壬��ͼ������������L�ĺϳ�·�ߡ�
��֪��R1��CHO+R2CH2��COOR3![]()
R1��CHO+R2NH2![]() R1��CH=N��R2
R1��CH=N��R2
��1���Լ�a��__��
��2��C����D�ķ�Ӧ������__��
��3��D����E�Ļ�ѧ����ʽ��___��
��4������G�Ļ�ѧ����ʽ��__��
��5��H�Ľṹ��ʽ��__��
��6��д����������������I��ͬ���칹��Ľṹ��ʽ__��
a.�Ƿ�ʽ�ṹ
b.�ܷ���������Ӧ
c.�����ϵ�һ�ȴ�����2��
d.1mol���л�������2mol�������Ʒ�Ӧ
��7��K�ķ���ʽ��C10H8O2��K�Ľṹ��ʽ��__��
��8����2-����ϩ������Ϊԭ�ϣ�ѡ�ñ�Ҫ�����Լ����ϳ�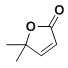 ��д���ϳ�·��__���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ������
��д���ϳ�·��__���ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ������
���𰸡�Ũ���ᣬŨ���� ȡ����ˮ�⣩��Ӧ 2 +O2
+O2![]() 2
2 +2H2O CH3COOH+CH3OH
+2H2O CH3COOH+CH3OH![]() CH3COOCH3+H2O
CH3COOCH3+H2O 



��������
 ��B�ڹ�������������������ȡ����Ӧ����
��B�ڹ�������������������ȡ����Ӧ����![]() �����ƿ�֪B��
�����ƿ�֪B��![]() ��A����������Ӧ����B��F�ķ���ʽ��C2H4O��F��״���Ũ���������·�Ӧ����G����F�����ᡢG�����������
��A����������Ӧ����B��F�ķ���ʽ��C2H4O��F��״���Ũ���������·�Ӧ����G����F�����ᡢG����������� �����R1��CHO+R2CH2��COOR3
�����R1��CHO+R2CH2��COOR3![]() ����֪HΪ
����֪HΪ![]() �����R1��CHO+R2NH2
�����R1��CHO+R2NH2![]() R1��CH=N��R2��
R1��CH=N��R2�� �� ���ƿ�֪E��
�� ���ƿ�֪E�� ��K�ķ���ʽ��C10H8O2��K��
��K�ķ���ʽ��C10H8O2��K�� ����
���� ���ƣ�D��
���ƣ�D�� ��
��
�������Ϸ�������1��A�Ǽױ���B��![]() ��A����������Ӧ����B�������Լ�a��Ũ���ᣬŨ���
��A����������Ӧ����B�������Լ�a��Ũ���ᣬŨ���
��2��C��![]() ��D��
��D�� ��C����
��C����
��3��D�� ��E��
��E�� ��D����E�Ǵ��Ĵ���������Ӧ�Ļ�ѧ����ʽ��2
��D����E�Ǵ��Ĵ���������Ӧ�Ļ�ѧ����ʽ��2 +O2
+O2![]() 2
2 +2H2O��
+2H2O��
��4��G���������������ͼ״�����Ũ���������·�Ӧ���������������Ӧ�Ļ�ѧ����ʽ��CH3COOH+CH3OH![]() CH3COOCH3+H2O��
CH3COOCH3+H2O��
��5�� �����R1��CHO+R2CH2��COOR3
�����R1��CHO+R2CH2��COOR3![]() ����֪HΪ
����֪HΪ![]() ��
��
��6��a.�Ƿ�ʽ�ṹ��˵������̼̼˫����b.�ܷ���������Ӧ��˵������ȩ����������� c.�����ϵ�һ�ȴ�����2�֣�˵��2��ȡ�����ڱ����Ķ�λ�� d.1mol���л�������2mol�������Ʒ�Ӧ��˵���Ǽ������������������I��ͬ���칹��Ľṹ��ʽ�� ��
��
��7�����R1��CHO+R2NH2![]() R1��CH=N��R2��
R1��CH=N��R2�� �� ���ƿ�֪E��
�� ���ƿ�֪E�� ������K�ķ���ʽ��C10H8O2����֪K��
������K�ķ���ʽ��C10H8O2����֪K�� ��
��
��8��2-����ϩ����ˮ�����ӳɷ�Ӧ���� ��
�� ˮ��Ϊ
ˮ��Ϊ ��
�� ��ͭ������������������Ϊ
��ͭ������������������Ϊ ��
��  �����ᷢ������Ӧ��Ӧ����
�����ᷢ������Ӧ��Ӧ���� ������R1��CHO+R2CH2��COOR3
������R1��CHO+R2CH2��COOR3![]() ��
�� �ڼ���ȵ�����������
�ڼ���ȵ����������� ���ϳ�·��Ϊ
���ϳ�·��Ϊ ��
��





