��Ŀ����
һ�������£������Ϊ10L���ܱ������У�1molX��1molY���з�Ӧ��2X��g����Y��g�� Z��g������60s�ﵽƽ�⣬����0��3molZ������˵����ȷ����
Z��g������60s�ﵽƽ�⣬����0��3molZ������˵����ȷ����
A��0��60s����X��Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0��001mol��L��1��s��1
B�������������Ϊ20L��Z��ƽ��Ũ�ȱ�Ϊԭ����
C��������ѹǿ��������Y��ת���ʼ�С
D���������¶ȣ�X���������������÷�Ӧ�Ħ�H>0
��ϰ��ϵ�д�
 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
�����Ŀ
11���ö��Ե缫���һ��ʱ������ʶ���ʣ�ࣩ���ס������ش����Ҽ�����������Һ��pH�仯������ͬ�������������������ķ�Ӧ��������ʵ����ֱ���ȵ��ǣ�������
| A | B | C | D | |
| �� �� | HCl��aq�� | CuCl2��aq�� | NaOH��aq�� | H2SO4��aq�� |
| �� �� | NaCl��aq�� | K2SO4��aq�� | CuSO4��aq�� | AgNO3��aq�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
9�����и�������еĸ���ԭ�ӣ����ߴ���ͬһƽ�棬������һ��ֱ���ϵ��ǣ�������
| A�� | CO2��HCl��HC��CCH3 | B�� | C6H6��C2H4��CO2 | C�� | C6H6��HCl��CH4 | D�� | C2H6��HCl��C2H2 |
6��ij��Ȼ��֬10g����1.8gNaOH������ȫ��������֪����֬1000g���д����⣬��������12g������ȫӲ�������ƶ�1mol����֬ƽ����̼̼˫����Ϊ��������
| A�� | 2mol | B�� | 3mol | C�� | 4mol | D�� | 5mol |
A~H��Ϊ������Ԫ�أ�A~F��Ԫ�����ڱ��е����λ������ͼ��ʾ��G����������Ԫ�ز���ͬһ���ڣ�H�Ƕ�������ԭ�Ӱ뾶��������Ԫ�ء���B��G��ɵ���̬�������ˮ��Һ�ʼ��ԡ�
A | B | C |
|
D |
| E | F |
��ش��������⣺
��1��д���ĵ���ʽ��ʵ������ȡ����Ļ�ѧ����ʽΪ��
��2��B��C��G������Ϊ1:1:5�γɵĻ�����Ļ�ѧ������Ϊ��
A�����Ӽ�
B�����Լ�
C���Ǽ��Լ�
��3�����õ���ʽ��ʾAE2���γɹ��̡�
��4�������ӷ��ű�ʾC��E��F��H�������ӵİ뾶�ɴ�С��˳��
��5����һ�����ӷ���ʽ����A��D�ǽ�����ǿ��ԭ��



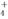 ��>c��Cl������c��OH����>c��H����
��>c��Cl������c��OH����>c��H����