��Ŀ����
SiO2+2C+2Cl
| ||
��1��12gC���뷴Ӧʱ����ת�Ƶ���Ŀ��______����2���ڸ÷�Ӧ�У���������______��
II����д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
��1��������ӡˢ��·��Ĺ����г�����ͭ���Ȼ�����Һ��Ӧ�����ӷ���ʽ______��
��2����������������̷�Ӧ����ȡ���۵�����̵Ļ�ѧ����ʽ______��
III��ij��ѧ��ȤС����Ӻ�������ȡ�⣬��������ʵ�飺�ɺ���
| ���� |
| ||
| ���� |
| ||
| ��ȡ��Һ |
��A��C��ѡ���ʵ���װ����գ�����ĸ����

��1�����˲���ʱ�õ���װ����______��
��2����I2��CCl4��Һ����ȡ����I2������CCl4�Ŀ���װ��______��
II����1��ͭ���Ȼ�����Һ��Ӧ����2FeCl2��CuCl2���ʴ�Ϊ��2Fe3++Cu=2Fe2++Cu2+��
��2���������ȷ�ұ��������ԭ��������������������Ӧ�õ�����������������������MnO2��Ӧ�Ļ�ѧ����ʽΪ4Al+3MnO2
| ||
�ʴ�Ϊ��4Al+3MnO2
| ||
III����1������ͼ���жϣ�C�ǹ���ʵ��װ��ʵ�飬�ʴ�Ϊ��C��
��2����CCl4�ӷ�����������ķ��������CCl4���ʴ�Ϊ��A��

 ��У����ϵ�д�
��У����ϵ�д����յķϾ�п�̸ɵ�ؾ���������õ��̷�(��MnO2��Mn(OH)2��Fe����Ȳ�ͺ�̿��)�����̷���ȡMnO2�IJ�������ͼ��ʾ��
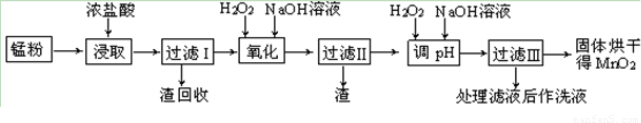
������ͼ��ʾ���貢�ο��������ݣ��ش��������⡣
�� �� | ��ʼ���� | ������ȫ |
Fe(OH)3 | 2.7 | 3.7 |
Fe(OH)2 | 7.6 | 9.6 |
Mn(OH)2 | 8.3 | 9.8 |
��1����������������Ũ�����ȡ�̷ۣ�������Һ�к���Mn2+��Fe2+�ȡ�MnO2��Ũ���ᷴӦ�����ӷ��̷���ʽ��?????????????????????????????????????????????????????? _��
��2�����ʱ������ʱ����̽����ʵ�Ӱ������ͼ��ʾ����ҵ���õ��ǽ�ȡ60 min�������ԭ����???????????????????????????????????????????????????? ��
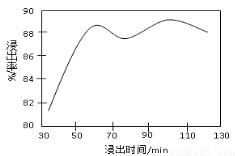
��3���̷۾�Ũ�����ȡ������I��ȥ�������ʺ�����Һ�м�������H2O2��Һ����������????????????????????????????????? ��
��4������I������Һ�������������NaOH��Һ����pHԼΪ5.1����Ŀ����?????????????????????????????????????????????? ��
��5��������������Һ��������H2O2��Һ������NaOH��Һ����pHԼΪ9��ʹMn2+ �����õ�MnO2����Ӧ�����ڷ���ʽΪ????????????????????????????????????????????????????? ��
��6����ҵ������KOH��MnO2Ϊԭ����ȡKMnO4����Ҫ�������̷��������У���һ����MnO2����KOH���飬��Ͼ��ȣ��ڿ����м������ۻ�����������������ȡK2MnO4���ڶ���Ϊ���K2MnO4��Ũ��Һ��ȡKMnO4��
�� ��һ����Ӧ�Ļ�ѧ����ʽΪ???????????????????????????????????????????????????????????? ��
�� ���K2MnO4��Ũ��Һʱ��������������ʵ������Ϊ???????????????????????????????????????? ��
 I���±���ʵ�����Ʊ�������й����ݣ�
I���±���ʵ�����Ʊ�������й����ݣ�
| ��� | ʵ������ | ʵ��ԭ�� | ����װ�� |
| �� | ������ | H2O2��O2 | |
| �� | �ư��� | NH4Cl��NH3 | |
| �� | ������ | HCl��Cl2 |
��2�����ݱ�������ʵ��ԭ����������װ����ѡ����ʵķ���װ�ã������������ϱ��Ŀո��У�����
��3�����������Ʊ�O2��װ���Ʊ�NH3����ѡ����Լ�Ϊ______��
��֪��NH3?H2O�ĵ��뷽��ʽΪ��NH3?H2O?NH+4+OH-����д��NH3����ˮ���γɵ�NH3?H2O�ĺ����ṹ______
��4���Ʊ�Cl2����8mol?L-1������100mL������12mol?L-1�����������ƣ�
����Ҫ12mol?L-1����������Ϊ______mL����ȷ��0.1mL��
��������ƿ��ʹ�÷����У����в�������ȷ����______����д��ţ���
A��ʹ������ƿǰ������Ƿ�©ˮ
B������ƿ������ˮϴ�������ô�����Һ��ϴ
C��������Һʱ����Ͳ��ȡŨ������ò���������������ƿ�У�������������ˮ���ӽ�����1cm��2cm�����ý�ͷ�ιܵμ�����ˮֱ����Һ�����ʹ��ͱ�����ƽ
D�����ݺ�Ǻ�ƿ������ʳָ��ס������һֻ����ָ��סƿ�ף�������ƿ��ת��ҡ�����
II����1�������£���֪0.1mol?L-1һԪ��HA��Һ��c��OH-��/c��H+��=1��10-8��
д������HA��NaOH��Һ��Ӧ�����ӷ���ʽʽ��______��
��2��t��ʱ����pH=2��ϡ�����pH=11��NaOH��Һ�������Ϻ���Һ�����ԣ�
����¶��£�t�棩����100mL 0.1mol?L-1��ϡH2SO4��Һ��100mL 0.4mol?L-1��NaOH��Һ��Ϻ���Һ����仯���Բ��ƣ�����Һ��pH=______��
 I���±���ʵ�����Ʊ�������й����ݣ�
I���±���ʵ�����Ʊ�������й����ݣ�