��Ŀ����
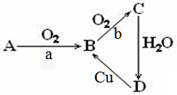
��12�֣�����ΪA��B��C��D�������ʵ�ת����ϵ��a��bΪ��Ӧ������

��1����AΪ���ʣ�aΪ��ȼ��bΪ���������ȣ���AΪ ����д��ѧʽ����
д��B��C�ķ���ʽ ��
��2����AΪ��̬�����B��Cת������Ҫ��������AΪ ����д��ѧʽ����
д��A��B��ѧ����ʽ ��
д��Cu+D��Һ��B�����ӷ���ʽ ��
��3����ij��ɫ����Y���������ȣ�Ͷ�뵽����ij����ɫ��ҺD�в�������������ɵĻ������X����X��������ʾʵ�飺

д��M��G���������ʵĻ�ѧʽ M G
���𰸡�
��1+2�֣� ��1��S��2SO2+O2=2SO3�����ȡ����������淴Ӧ����
��1+2+2�֣���2��NH3��4NH3+5O2=4NO+6H2O�����ȡ���������3Cu+8H++2NO3-=3Cu2++2NO��+4H2O
��2+2�֣� ��3��NO��CO2����1��0�֣��� Cu(NO3)2
��������

��ϰ��ϵ�д�
�����Ŀ