��Ŀ����
�����ѧʽΪNa2CO3����һ����Ҫ�Ļ���ԭ�ϡ��ִ��������������ֹ��գ�
һ�������Ƽ����ʳ��Ϊԭ���Ƽ�÷���������
�����Ȼ��������ᷴӦ�������ƣ�2NaCl+H2SO4=Na2SO4+2HCl��
���ý�̿��ԭ�����Ƶ����ƣ�Na2SO4+4C=Na2S+4CO��
����������ʯ��ʯ��Ӧ��̼���ƣ�Na2S+CaCO3=Na2CO3+CaS
�������������ά�Ƽ����ʳ�Ρ�����������̼Ϊԭ�ϣ��䷴ӦҲ���������У�
��NH3+CO2+H2O=NH4HCO3
��NH4HCO3+NaCl=NaHCO3+NH4Cl
��2NaHCO3=Na2CO3+CO2��+H2O
���������Ƽ������������ʳ��ˮ��ͨ�백������ͨ�������̼������̼�����ƣ��ټ���ϸ��ĩ����ͬ����ЧӦ�������Ȼ���ܽ��ͻȻ���ͣ���ʳ�ε��ܽ�ȱ仯���������Ȼ��������ʳ�β����������ð����ͺ�ͨ������̼�������������NaHCO3��NH4Cl���÷������Ĵ�������������������ѩ��
��1��ͨ�����ַ����ıȽϣ������Ƽ���յ�ȱ���� (д����)��
��2��������յ���ѭ�����õ������� (�ѧʽ)����Ʒ�ĸ�����NH4Cl�ȿ����������ֿ����������ɰ�����д��NH4Cl����ʯ�ҷ�Ӧ�Ļ�ѧ����ʽ ��
��3�������Ƽ��Ӧ�ķ���ʽΪ ��
��4��Ϊʲô�����Ƽ����������ʳ��ˮ��ͨ�백������ͨ�������̼�������� (д����)��
��5�������Ƽ��Ʒ�����к���̼�����ơ�����ü��ȷֽ�ķ����ⶨ������̼�����Ƶ�������������m1��ʾ����ǰ������Ʒ��������m2��ʾ���Ⱥ���������������̼�����Ƶ����������ɱ�ʾΪ�� ��
��1������ʱ��Ҫ���£�������豸��ʴ���أ�CaS�����ﳤ�ڶѻ��������磻�ɱ��ϸߡ�(4�֣�����������������2�֣����������𰸾�����)
��2��CO2(1��) 2NH4Cl+CaO 2NH3��+CaCl2+H2O(2��)
2NH3��+CaCl2+H2O(2��)
��3��NaCl+CO2+NH3+H2O=NaHCO3��+NH4Cl(2��)
��4��CO2��ˮ�е��ܽ�Ƚ�С�������ʵͣ���ͨ��CO2��֤ȫ������NaHCO3(4�֣�����������������2�֣����������𰸾�����)
��5�� (2��)
(2��)
���������������1�������Ƽ���յ�ȱ���н�̿��ԭ������ʱ��Ҫ���£�������豸��ʴ���أ�CaS�����ﳤ�ڶѻ��������磻�ɱ��ϸߵȣ�
��2���ӻ�ѧ����ʽ�еó�������յ���ѭ�����õ�������CO2���Ȼ������ʯ�ҷ�Ӧ���ɰ������Ȼ��ơ�ˮ����ѧ����ʽΪ2NH4Cl+CaO 2NH3��+CaCl2+H2O��
2NH3��+CaCl2+H2O��
��3�������Ƽ��ԭ������ʳ��ˮ��ͨ�백������ͨ�������̼������̼�����ƣ����Ի�ѧ����ʽΪNaCl+CO2+NH3+H2O=NaHCO3��+NH4Cl ��
��4����Ϊ������̼��ˮ�е��ܽ�Ƚ�С����������̼�����ƣ�������ͨ�백����ʹ��Һ�ʼ��ԣ���ͨ�������̼�����������̼���������Ӷ�����̼�����ƣ�
��5��̼�����������ֽ⣬����̼���ơ�ˮ�Ͷ�����̼�����ոù���Ϊ̼���ƣ������������ᣨm1-m2��g��ÿ����168g̼�����ƣ�������������62g�����Թ�����̼�����Ƶ�������168��m1-m2��/62g,������Ʒ��̼�����Ƶ�����������168��m1-m2��/62/m= ��
��
���㣺���鹤ҵ�ƴ���ķ�������ѧ����ʽ����д�����������ļ��㣬�Թ��յķ���

10��ʱ����NaHCO3������Һ����ø���Һ��pH�������±仯��
| �¶ȣ��棩 | 10 | 20 | 30 | ������к���ȴ��50�� |
| pH | 8��3 | 8��4 | 8��5 | 8��8 |
��ͬѧ��Ϊ������Һ��pHֵ���ߵ�ԭ����HCO3����ˮ��̶����ʼ�����ǿ���÷�Ӧ�����ӷ���ʽΪ ��
��ͬѧ��Ϊ����ҺpH���ߵ�ԭ����NaHCO3���ȷֽ⣬������Na2CO3�����ƶ�Na2CO3��ˮ��̶� ������ڡ���С�ڡ���NaHCO3��
��ͬѧ��Ϊ�ס��ҵ��ж϶�����֡�����Ϊ��___________________________
��1��ֻҪ�ڼ�����е���Һ�м����������Լ�X���������������� ����ס����ҡ����ж���ȷ���Լ�X�� ��
A��Ba(OH)2��Һ B��BaCl2��Һ C��NaOH��Һ D������ʯ��ˮ
��2�������Ⱥ����Һ��ȴ��10�棬����Һ��pH����8��3���� ����ס����ҡ����ж���ȷ��
��3�������£��ڲ��PH������9��NaOH��Һ��Na2CO3��Һ�У���ˮ�����OH��Ũ�ȷֱ�Ϊamol /L��bmol /L����a��b�ı�ֵ= ��
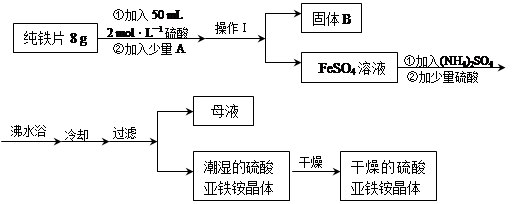
�Ի�����Ϊԭ��������������������к�Fe2O3��SiO2��Al2O3��MgO�ȡ�ʵ����ģ�ҵ���������Ʊ����죨Fe2O3�����������£�
��1���������ijɷ�������������������� ��д�����ܹ���Fe2O3��ϡ���ᷴӦ�����ӷ�Ӧ����ʽ ��
��2�����������У�Ϊ��ȷ������Ĵ��ȣ�����������Ҫ������Һ��pH�ķ�Χ�� ������������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 |
��3������A����Ҫ�ɷ�Ϊ ����ҺB���Ի��յ�������____________��
��4������ϴ�ӹ��̵�ʵ����� ��
��5����֪����������Ϊw kg�����������Ʊ������У���Ԫ�����25%�����յõ����������Ϊm kg����ԭ������������Ԫ����������Ϊ ��������������ʽ��ʾ����