��Ŀ����
����Ŀ��(1)��֪��HCN�ĵ��볣��Ka��4.9��10��10��H2S�ĵ��볣��Ka1��1.3��10��7��Ka2��7.0��10��15����NaCN��Һ��ͨ��������H2S���壬��Ӧ�Ļ�ѧ����ʽΪ__________��
(2)��һ�������¿��ü״���CO��Ӧ���ɴ�������CO��Ⱦ�������£���a mol��L��1�Ĵ�����b mol��L��1 Ba(OH)2��Һ�������ϣ���ַ�Ӧ����Һ�д���2c(Ba2��)��c(CH3COO��)����û����Һ�д���ĵ��볣��Ka��_____(�ú�a��b�Ĵ���ʽ��ʾ)��
(3)��֪��25 ��ʱ��H2C2O4�ĵ��볣��Ka1��5.9��10��2��Ka2��6.4��10��5����25 ��ʱ��0.1 mol��L��1NaHC2O4��____�ԣ�������___���������Һ�м���һ����NaOH���壬ʹc(HC2O4-)��c(C2O42-)�����ʱ��Һ��c(H��)=____��
���𰸡�NaCN+H2S=HCN+NaHS ![]() ��10-7L��mol��1 �� HC2O4��ˮ�ⳣ��С�ڵ��볣����HC2O4���ĵ���̶ȴ���ˮ��̶� 6.4��10-5
��10-7L��mol��1 �� HC2O4��ˮ�ⳣ��С�ڵ��볣����HC2O4���ĵ���̶ȴ���ˮ��̶� 6.4��10-5
��������
��1����֪��25��ʱ��HCN�ĵ��볣��K=4.9��10-10��H2S�ĵ��볣��K1=1.3��10-7��K2=7.0��10-15��������H2S��HCN��HS��������ǿ���Ʊ��������ͨ��H2S������Ӧ�Ļ�ѧ����ʽ��
��2����Һ������������Ũ�ȼ���һ�룬�������ƽ�ⳣ����Ũ���أ���ϸ�����㣻
��3����Һ�д����ŵ���ƽ��HC2O4��![]() H��+C2O42����ˮ��ƽ�� HC2O4��+H2O
H��+C2O42����ˮ��ƽ�� HC2O4��+H2O![]() H2C2O4+OH������ΪHC2O4���ĵ���̶ȴ���ˮ��̶ȣ�������Һ�����ԣ�NaHC2O4��Һ�����ԣ���Һ��c��H������c��HC2O4����������ƽ�⣺HC2O4��
H2C2O4+OH������ΪHC2O4���ĵ���̶ȴ���ˮ��̶ȣ�������Һ�����ԣ�NaHC2O4��Һ�����ԣ���Һ��c��H������c��HC2O4����������ƽ�⣺HC2O4��![]() H��+C2O42����H2O
H��+C2O42����H2O![]() H��+OH�������ݵ��볣���������㡣
H��+OH�������ݵ��볣���������㡣
��1��25��ʱ��HCN�ĵ��볣��K=4.9��10-10��H2S�ĵ��볣��K1=1.3��10-7��K2=7.0��10-15��������H2S��HCN��HS��������ǿ���Ʊ����ᣬ��NaCN��Һ��ͨ��������H2S���壬�����ķ�ӦΪNaCN+H2S=HCN+NaHS��
��2����Ӧƽ��ʱ��2c��Ba2����=c��CH3COO����=bmol��L��1���ݵ���غ㣬��Һ��c��H����=c��OH����=10-7mol��L��1����Һ�����ԣ��������ƽ�ⳣ�����ݵ��뷽��ʽд��K=![]() =
=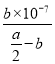 =
=![]() ��10-7���ú�a��b�Ĵ���ʽ��ʾ�û����Һ�д���ĵ��볣��Ϊ
��10-7���ú�a��b�Ĵ���ʽ��ʾ�û����Һ�д���ĵ��볣��Ϊ![]() ��10-7L��mol��1��
��10-7L��mol��1��
��3����Һ�д����ŵ���ƽ��HC2O4��![]() H��+C2O42����ˮ��ƽ�� HC2O4��+H2O
H��+C2O42����ˮ��ƽ�� HC2O4��+H2O![]() H2C2O4+OH����ˮ���ƽ�ⳣ����
H2C2O4+OH����ˮ���ƽ�ⳣ����![]() =
=![]() <Ka2��6.4��10��5����HC2O4���ĵ���̶ȴ���ˮ��̶ȣ�������Һ�����ԣ��������Һ�м���һ����NaOH���壬ʹc��HC2O4����=c��C2O42��������c��H����==
<Ka2��6.4��10��5����HC2O4���ĵ���̶ȴ���ˮ��̶ȣ�������Һ�����ԣ��������Һ�м���һ����NaOH���壬ʹc��HC2O4����=c��C2O42��������c��H����==![]() = Ka2=6.4��10-5��
= Ka2=6.4��10-5��

 �¿α�������������ҵ�������γ�����ϵ�д�
�¿α�������������ҵ�������γ�����ϵ�д�






