��Ŀ����
����ʯ����Կ�����MgO��Fe2O3��Al2O3��SiO2��ɣ�������ʯ��ȡ��ʽ̼��þ��ʵ�鲽������

| �������� | Fe��OH��3 | Al��OH��3 | Mg��OH��2 |
| ��ʼ����pH | 1.5 | 3.3 | 9.4 |
��2�����Т����ʱ��������ҺpH=7-8���й��������������pH������Ca��OH��2���ܹ�������Ca��OH��2�������ܻᵼ��______�ܽ⡢______������
��3���ӳ��������A����ȡ��ɫ�����������ϣ����������A�м���______ ���������ʵĻ�ѧʽ����Ȼ��______ ��������дʵ��������ƣ���
��4������ѭ��ʹ�ã��ܽ�Լ��Դ������ʵ���У�����ѭ��ʹ�õ�������______����д���ʻ�ѧʽ����
��5�������һ��ʵ�飬ȷ����ƷaMgCO3?bMg��OH��2?cH2O��a��b��c��ֵ����֪��Ϊ��������������������ʵ�鲽�裨�����Լ���Ũ���ᡢ��ʯ�ң�������Ʒ�������ڸ��·ֽ⡡��______ ��______�� �ݳƳ�MgO��������
��2���ɣ�1��֪����ʱ��Һ�г���Mg2+�⣬������Fe3+��Al3+���ʣ����ȥFe3+��Al3+��������ʧMg2+����Fe3++3H2O?Fe��OH��3+3H+��Al3++3H2O?Al��OH��3+3H+������ƽ���ƶ���ԭ��������H+ʹ����ˮ��ƽ��������Ӧ�����ƶ�����ȥFe3+��Al3+����Ca��OH��2�轫��Һ��pH������7��8����pH���ߣ��ᵼ�����ɵ�Al��OH��3������ӦAl��OH��3+OH-=AlO2-+2H2O�ܽ⣬Mg��OH��2��pHΪ9.4ʱ��ʼ���������Լ�����ǿMg��OH��2�������ͬʱMg2+Ҳ��ת��Ϊ��������ʧ��
�ʴ�Ϊ��Al��OH��3��Mg��OH��2��
��3����ʵ�鲽��ͼ֪�����������ΪFe��OH��3��Al��OH��3����ɫ�������dz�����Fe��OH��3�ֽ��õ���Fe2O3�����Ե��ȼӼ��Al��OH��3��������ӦΪ��Al��OH��3+NaOH=NaAlO2+2H2O��Ȼ�����ϴ�����ռ��ɣ��ʴ�Ϊ��NaOH�����ˡ�ϴ�ӡ����գ�
��4����ʵ�鲽��ͼ֪������ʵ���У��������ͨ������̼������̼��ƣ�̼��Ʒֽ�ɵõ�������̼����Ϊ�ڢ�ԭ�ϣ���ʽ̼��þ�ֽ�õ�CO2������ѭ��ʹ�õ�������CaCO3��CO2���ʴ�Ϊ��CaCO3��CO2��
��5������Ũ�������ˮ�Բ��Ũ��������ز��ˮ���������������ü�ʯ������CO2ǰ����������������̼������
�ʴ�Ϊ���۲��ˮ����������������Ũ��������أ����ܲ��CO2��������������ʯ�ҵ����أ���
��������1������ʯ����Կ���MgO��Fe2O3��Al2O3��SiO2��ɣ�����ʯ�������ܽ��MgO��Fe2O3��Al2O3��HCl��Ӧ�ܽ⣬��SiO2��HCl����Ӧ�������ܽ⣻
��2�����������ƹ���ʱ����Һ������ǿ��Al��OH��3���ܽ⣬���������������pH���пɿ�����Mg��OH��2��pHΪ9.4ʱ��ʼ���������Լ�����ǿMg��OH��2�������
��3����ɫ������ΪFe2O3��Ӧ�Ƚ����к��е�����Al��OH��3��ȥ����ȥAl��OH��3�ķ�����������������ǿ������ʣ���4���˹�����CO2�ǿ����ظ�ʹ�õģ�
��5��ȷ����ƷaMgCO3?bMg��OH��2?cH2O��a��b��c��ֵ����Ҫ�ⶨ�������Ǣ���Ʒ��������MgO������������CO2�����������������������ˮ��������
������������Ҫ������Ԫ�ػ������н��������������ʣ�����ʱ��������֪���ʼ�ķ�Ӧԭ�����������������ʵ�����ƣ�

 ��Կ���Ծ�ϵ�д�
��Կ���Ծ�ϵ�д�����ʯ����Կ�����MgO��Fe2O3��Al2O3��SiO2��ɡ�������ʯ��ȡ��ʽ̼��þ��ʵ�鲽�����£�
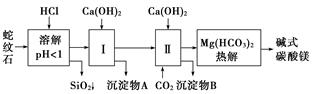
(1)����ʯ��������ܽ����Һ�����Mg2���⣬�����еĽ���������__________________��
(2)���Т����ʱ��������ҺpH��7��8(�й��������������pH���±�)��
|
�������� |
Fe(OH)3 |
Al(OH)3 |
Mg(OH)2 |
|
��ʼ����pH |
1.5 |
3.3 |
9.4 |
Ca(OH)2���ܹ�������Ca(OH)2�������ܻᵼ��________�ܽ⡢________������
(3)�ӳ��������A����ȡ��ɫ�����������ϣ����������A�м���_____________________ ___________________________________________________(�������ʵĻ�ѧʽ)��
Ȼ��______________________________________________________________________(������дʵ���������)��
(4)����ѭ��ʹ�ã��ܽ�Լ��Դ������ʵ���У�����ѭ��ʹ�õ�������________(��д���ʻ�ѧʽ)��
(5)�����һ��ʵ�飬ȷ����ƷaMgCO3��bMg(OH)2��cH2O��a��b��c��ֵ������������ʵ�鲽��(�����Լ���Ũ���ᡢ��ʯ��)��
����Ʒ����
�ڸ��·ֽ�
��________________________________________________________________________
��________________________________________________________________________
��MgO����
(6)18.2 g��Ʒ��ȫ�ֽ����6.6 g CO2��8.0 g MgO���ɴ˿�֪����Ʒ�Ļ�ѧʽ�У�
a��________��b��________��c��________��




