��Ŀ����
��֪AΪ����BΪ���ĺ���������ɵ����ʵ����� A �� B ��ɵĻ���� 0.05mol ��0.125mol ��������ǡ����ȫȼ�գ����� 0.1mol �� CO2 �� 0.1mol H2O����ͨ������ش��������⣺
��1���ӷ���ʽ�ĽǶȿ��������ʵ���A �� B ��ɵĻ����ƽ����ɿ��û�ѧʽ��ʾΪ______��
��2����ȡһ������A��B��ȫȼ�գ��������������ʵ����Ȼ�ϣ������ʵ���֮��һ������
����������һ������A��B�Ľṹ��ʽ�ֱ�Ϊ��A______ B______
�������ɵ�CO2 �� H2O �����ʵ���һ������A��B�Ľṹ��ʽΪ��A______ B______��
��1���ӷ���ʽ�ĽǶȿ��������ʵ���A �� B ��ɵĻ����ƽ����ɿ��û�ѧʽ��ʾΪ______��
��2����ȡһ������A��B��ȫȼ�գ��������������ʵ����Ȼ�ϣ������ʵ���֮��һ������
����������һ������A��B�Ľṹ��ʽ�ֱ�Ϊ��A______ B______
�������ɵ�CO2 �� H2O �����ʵ���һ������A��B�Ľṹ��ʽΪ��A______ B______��
��A��B�Ļ����0.05molȼ������0.1 molCO2��0.1molH2O�Լ�����������֪A��B�Ļ����ƽ����ɷ���ʽΪC2H4O������AΪ����BΪ���ĺ����������A��BΪ�����ʵ���������ƽ��ֵ���壨��A��B��̼ԭ����ȷ���������ֿ��ܣ�һ�����������̼ԭ������Ϊ2����һ�����һ��̼ԭ��������2��һ��̼ԭ����С��2����A��B����Ͽ��������֣��ֱ���CH4��C3H4O2��C2H4��C2H4O2��C2H2��C2H6O2��C2H6��C2H2O2��C3H6��CH2O2��
��1��������ȼ�շ�Ӧ���ݿ�֪��1 mol�����+2.5 molO2��2 molCO2+2mol H2O���������غ�ĽǶȵĿ�֪������ƽ������ʽΪC2H4O���ʴ�Ϊ��C2H4O��
��2��������AΪ����BΪ���ĺ����������B�бغ�2��Oԭ�ӣ��������������ʵ����Ȼ�ϣ������ʵ���֮��һ��ʱ������̼ԭ������Ϊ2��ӦΪCH��CH��
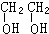
��
�ʴ�Ϊ��CH��CH��
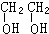
��
�������ɵ�CO2 �� H2O �����ʵ���һ���������������к��е�C��Hԭ������ͬ��ӦΪCH2=CH2��CH3COOH��
�ʴ�Ϊ��CH2=CH2��CH3COOH��
��1��������ȼ�շ�Ӧ���ݿ�֪��1 mol�����+2.5 molO2��2 molCO2+2mol H2O���������غ�ĽǶȵĿ�֪������ƽ������ʽΪC2H4O���ʴ�Ϊ��C2H4O��
��2��������AΪ����BΪ���ĺ����������B�бغ�2��Oԭ�ӣ��������������ʵ����Ȼ�ϣ������ʵ���֮��һ��ʱ������̼ԭ������Ϊ2��ӦΪCH��CH��
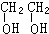
��
�ʴ�Ϊ��CH��CH��
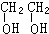
��
�������ɵ�CO2 �� H2O �����ʵ���һ���������������к��е�C��Hԭ������ͬ��ӦΪCH2=CH2��CH3COOH��
�ʴ�Ϊ��CH2=CH2��CH3COOH��

��ϰ��ϵ�д�
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
�����Ŀ



