��Ŀ����
�ϳɰ���ҵ����Ҫ�Ļ�����ҵ����ͳ�ϳɰ��ķ�����N2��g��+3H2��g��?2NH3��g������H=92.4kJ��mol-1��Ӱ��ϳɰ���ҵ����Ч�ʵ�������Ҫ���¶ȡ�ѹǿ��������ԭ������Ⱥ�������װ���е����٣�
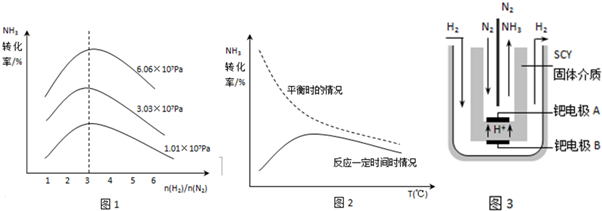
��1���÷�Ӧ���ȣ������¶ȶԷ�Ӧת���ʲ���������ҵ������ͨ����420-500�������½��и÷�Ӧ���������� ��
��2��ͼ1��ѹǿ��ԭ������ȶԷ�Ӧת����Ӱ���о������ͼ�н�ʾ�Ĺ����У� �� ��
��3��ͼ2�Ƿ�Ӧת�������¶ȹ�ϵͼ���Ķ�ͼ����Ϣ��˵��Ϊʲôʵ��������������װ���е�������Ӱ��ת���ʵ���Ҫ���أ�
��4��2001��ϣ����ѧ�����õ�ⷴӦ�ϳɰ�ȡ�óɹ���ʵ��װ����ͼ3����������ܴ���H+�����ٵ缫A�ǵ��ص� ��������������������ü��ϵĵ缫��Ӧʽ�� ��
��5�����о������ڳ��¡���ѹ�����������£�N2�ڴ���������ˮ������Ӧ����NH3��2N2+6H2O?4NH3+3O2��
��N2��g��+3H2O��1��=2NH3��g��+
O2��g����H=+765.2kJ?mol-1
��Ӧ2N2��g��+6H2O��l��?4NH3��g��+3O2��g���ġ�H= kJ?mol-1��

��1���÷�Ӧ���ȣ������¶ȶԷ�Ӧת���ʲ���������ҵ������ͨ����420-500�������½��и÷�Ӧ����������
��2��ͼ1��ѹǿ��ԭ������ȶԷ�Ӧת����Ӱ���о������ͼ�н�ʾ�Ĺ����У�
��3��ͼ2�Ƿ�Ӧת�������¶ȹ�ϵͼ���Ķ�ͼ����Ϣ��˵��Ϊʲôʵ��������������װ���е�������Ӱ��ת���ʵ���Ҫ���أ�
��4��2001��ϣ����ѧ�����õ�ⷴӦ�ϳɰ�ȡ�óɹ���ʵ��װ����ͼ3����������ܴ���H+�����ٵ缫A�ǵ��ص�
��5�����о������ڳ��¡���ѹ�����������£�N2�ڴ���������ˮ������Ӧ����NH3��2N2+6H2O?4NH3+3O2��
��N2��g��+3H2O��1��=2NH3��g��+
| 3 | 2 |
��Ӧ2N2��g��+6H2O��l��?4NH3��g��+3O2��g���ġ�H=
��������1���¶�Խ�ߣ���Ӧ����Խ�죬����ƽ������ʱ��Խ�̣�
��2���������߱仯���Ƽ��ݺ�����ĺ������������
��3����������Ӱ�췴Ӧ���ͣ��ʱ�䣬�Ӷ�Ӱ��ת���ʣ�
��4��������������ʧ���ӷ���������Ӧ�������ϵõ��ӷ�����ԭ��Ӧ��
��5�����ݸ�˹���ɼ��㣮
��2���������߱仯���Ƽ��ݺ�����ĺ������������
��3����������Ӱ�췴Ӧ���ͣ��ʱ�䣬�Ӷ�Ӱ��ת���ʣ�
��4��������������ʧ���ӷ���������Ӧ�������ϵõ��ӷ�����ԭ��Ӧ��
��5�����ݸ�˹���ɼ��㣮
����⣺��1���÷�Ӧ�Ƿ��ȷ�Ӧ�������¶�ƽ�����淴Ӧ�����ƶ������¶�Խ�߷�Ӧ����Խ�죬����ƽ������ʱ��Խ�̣������������ڣ��ʴ�Ϊ����߷�Ӧ���ʣ������������ڣ�
��2������ͼ��֪��ѹǿ�ƶ���������ת����Խ������������ȱ�֪����ֵԼ����3ʱ��ת������ߣ�
�ʴ�Ϊ��ѹǿԽ��ת����Խ�ߣ�ԭ�������Լ����3ʱת������ߣ�
��3��ʵ������Ϊ�����������ϳ����ڷ�Ӧ��ϵδ�ﵽƽ�⼴�ų�����ʱ����������Ӱ�췴Ӧ��ķ�Ӧʱ�䣬�Ӷ�Ӱ�췴Ӧת���ʣ�
�ʴ�Ϊ��ʵ������Ϊ�����������ϳ����ڷ�Ӧ��ϵδ��ƽ�⼴�ų����⣬��������Ӱ�췴Ӧ���ڷ�Ӧ����ͣ��ʱ�䣬�Ӷ�Ӱ�췴Ӧת���ʣ�
��4������ͼ֪�������ϵ����õ��Ӻ������ӷ�Ӧ���ɰ����������ٵ缫Ϊ��������缫��ӦʽΪN2+6e-+6H+=2NH3��
�ʴ�Ϊ������N2+6e-+6H+=2NH3��
��5��N2��g��+3H2O��1��=2NH3��g��+
O2��g����H=+765.2kJ?mol-1����2N2��g��+6H2O��l��?4NH3��g��+3O2��g���ġ�H=��+765.2kJ?mol-1����2=+1530.0kJ��mol-1��
�ʴ�Ϊ��+1530.0��
��2������ͼ��֪��ѹǿ�ƶ���������ת����Խ������������ȱ�֪����ֵԼ����3ʱ��ת������ߣ�
�ʴ�Ϊ��ѹǿԽ��ת����Խ�ߣ�ԭ�������Լ����3ʱת������ߣ�
��3��ʵ������Ϊ�����������ϳ����ڷ�Ӧ��ϵδ�ﵽƽ�⼴�ų�����ʱ����������Ӱ�췴Ӧ��ķ�Ӧʱ�䣬�Ӷ�Ӱ�췴Ӧת���ʣ�
�ʴ�Ϊ��ʵ������Ϊ�����������ϳ����ڷ�Ӧ��ϵδ��ƽ�⼴�ų����⣬��������Ӱ�췴Ӧ���ڷ�Ӧ����ͣ��ʱ�䣬�Ӷ�Ӱ�췴Ӧת���ʣ�
��4������ͼ֪�������ϵ����õ��Ӻ������ӷ�Ӧ���ɰ����������ٵ缫Ϊ��������缫��ӦʽΪN2+6e-+6H+=2NH3��
�ʴ�Ϊ������N2+6e-+6H+=2NH3��
��5��N2��g��+3H2O��1��=2NH3��g��+
| 3 |
| 2 |
�ʴ�Ϊ��+1530.0��
���������⿼���˻�ѧ��Ӧƽ����й�֪ʶ���漰��˹���ɡ��缫��Ӧʽ����д��ͼ�������֪ʶ�㣬ͼ�������ע���ݺ����ꡢ���߱仯���Ƶ�֪ʶ�㣬�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 2NH3(g)����H =" -92.2" kJ��mol-1��
2NH3(g)����H =" -92.2" kJ��mol-1��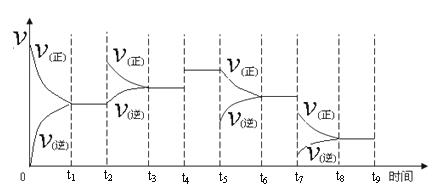
 H2(g) + CO(g) ��H =" +131.3" kJ/mol
H2(g) + CO(g) ��H =" +131.3" kJ/mol 2NH3(g)����H =" -92.2" kJ��mol-1��
2NH3(g)����H =" -92.2" kJ��mol-1��
 H2(g)+I2(g) �ﵽƽ���ƽ��ʱI2��Ũ��c(I2)A c(I2)B ��ƽ��ʱHI�ķֽ��ʦ�A ��B ��ƽ��ʱH2�ڻ�������е��������A B ����д��������������������
H2(g)+I2(g) �ﵽƽ���ƽ��ʱI2��Ũ��c(I2)A c(I2)B ��ƽ��ʱHI�ķֽ��ʦ�A ��B ��ƽ��ʱH2�ڻ�������е��������A B ����д��������������������