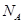��Ŀ����
������Ca(OH)2����ˮ�ﵽ����ʱ��������ƽ�⣺Ca(OH)2(s) Ca2++2OH��������ʯ��ˮ���ܶ�Ϊd g/cm3����ҺpH��12�������й������У�����ȷ����
Ca2++2OH��������ʯ��ˮ���ܶ�Ϊd g/cm3����ҺpH��12�������й������У�����ȷ����
 Ca2++2OH��������ʯ��ˮ���ܶ�Ϊd g/cm3����ҺpH��12�������й������У�����ȷ����
Ca2++2OH��������ʯ��ˮ���ܶ�Ϊd g/cm3����ҺpH��12�������й������У�����ȷ����| A��������50�棬��Һ��Ca2+��Ũ������ |
B�����¶���Ca(OH)2���ܽ��Ϊ (g/100gH2O) (g/100gH2O) |
| C���ñ���ʯ��ˮ�У�ˮ�������OH����Ũ��Ϊ10��12mol/L |
D��������ʯ�Ҳ��ָ������º� ֵ���� ֵ���� |
A
���������A���������Ƶ��ܽ�����¶ȵ����߶����ͣ���˼�����50�棬��Һ��Ca2+��Ũ�ȼ�С��A����ȷ��B����Һ��pH��12������Һ����������Ũ����0.01mol/L�������������Ƶ�Ũ����0.01mol��2��0.005mol/L��������Һ��1L�����������Ƶ����ʵ�����0.005mol��������0.37g�������ܼ�ˮ��������1000d��0.37g�������������Ƶ��ܽ����S��
 ��B��ȷ��C������������ǿ������ˮ�ĵ��룬��ñ���ʯ��ˮ�У�ˮ�������OH����Ũ�Ⱦ�����Һ��������Ũ��Ϊ10��12mol/L��C��ȷ��D���¶Ȳ��䣬���ӵ�Ũ�Ȳ��䣬��˱�ֵ
��B��ȷ��C������������ǿ������ˮ�ĵ��룬��ñ���ʯ��ˮ�У�ˮ�������OH����Ũ�Ⱦ�����Һ��������Ũ��Ϊ10��12mol/L��C��ȷ��D���¶Ȳ��䣬���ӵ�Ũ�Ȳ��䣬��˱�ֵ ���䣬D��ȷ����ѡA��
���䣬D��ȷ����ѡA��
��ϰ��ϵ�д�
�����Ŀ