��Ŀ����
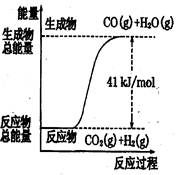
������ͼ��ʾ���Ȼ�ѧ����ʽ�� �� ��
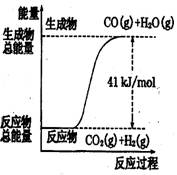
A��CO+H2O=CO2+H2����H=+41 kJ/mol
B��CO(g)+H2O(g)=CO2(g)+H2(g);����H= −41 kJ/mol
C��CO2(g)+H2(g)=CO(g)+H2O(g)����H=+41 kJ/mol
D��CO2(g)+H2(g) =CO(g)+H2O(g)����H=−41 kJ/mol
������������ͼ����жϷ�Ӧ������������������������������Ӧ�����ȷ�Ӧ��B��D����ȷ����ѡ��A��û�б������ʵ�״̬���Ǵ���ģ�������ȷ�Ĵ���C��
���𰸡�
C

��ϰ��ϵ�д�
�����Ŀ
������ͼ��ʾ���Ȼ�ѧ����ʽ�� �� ��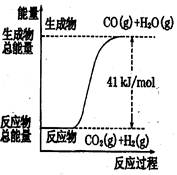
| A��CO+H2O=CO2+H2����H="+41" kJ/mol |
| B��CO(g)+H2O(g)=CO2(g)+H2(g);����H= ?41 kJ/mol |
| C��CO2(g)+H2(g)=CO(g)+H2O(g)����H="+41" kJ/mol |
| D��CO2(g)+H2(g) =CO(g)+H2O(g)����H=?41 kJ/mol |
 ��1��������һ�ָ�Ч��������Դ��0.25mol������ȫȼ������Һ̬ˮʱ���ų�222.5kJ�����������ȼ���ȵ��Ȼ�ѧ��Ϊ
��1��������һ�ָ�Ч��������Դ��0.25mol������ȫȼ������Һ̬ˮʱ���ų�222.5kJ�����������ȼ���ȵ��Ȼ�ѧ��Ϊ