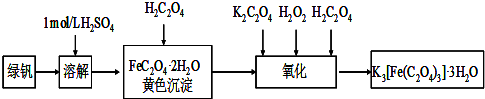
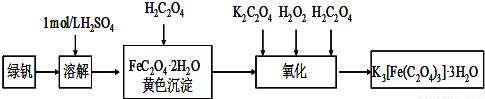
��Ŀ����
ijʵ��С��Ϊ�о��������ȡ�Ͳ�������ʣ���������ʵ�顣
ʵ��I:�Ʊ�����
ʵ������������������ˮ��Һ�Ʊ������װ����ͼ��ʾ(���ȡ�����������̶�װ�þ�����ȥ����ʵ��������£�
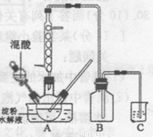
�ٽ�һ�����ĵ���ˮ��Һ��������ƿ��
�ڿ��Ʒ�ӦҺ�¶���55〜600C�����£��߽�������μ�һ�����������������Ļ��� (65%HNO3��98%H2S04��������Ϊ2 :1.5)��Һ
�۷�Ӧ3h���ң���ȴ�����˺����ؽᾧ�ò��ᾧ�塣
������������ˮ��Һ�����пɷ������з�Ӧ��
C6H12O6��12HNO3��3H2C2O4��9NO2����3NO����9H2O
C6H12O6��8HNO3��6CO2����8NO����10H2O
3 H2C2O4��2HNO3��6CO2����2NO����4H2O
(1)��������Ƿ�ˮ����ȫ�����õ��Լ�Ϊ________��
(2)ʵ����������μӹ��죬�����²�������½�����ԭ����________��
ʵ��II �����ᾧ���нᾧˮ�ⶨ
���ᾧ��Ļ�ѧʽ�ɱ�ʾΪH2C2O4 • xH2O,Ϊ�ⶨx��ֵ,��������ʵ�飺
�ٳ�ȡ6.3gij���ᾧ�����100.0mL��ˮ��Һ��
��ȡ25.00mL������Һ������ƿ�У���������ϡH2SO4����Ũ��Ϊ0. 5mol/L��KMnO4��Һ�ζ����ζ��յ�ʱ����KMnO4 �����Ϊ10.00mL���ش�����������
��3��д��������Ӧ�����ӷ���ʽ________________��
��4������x=________��
��5���ζ�ʱ�������ַ�Ӧ���ʿ�ʼ�����������ӿ죬���ܵ�ԭ����________��
ʵ��III:����ȶ���
�������ϣ����ᾧ��(H2C2O4 •xH20),1000C��ʼʧˮ��100.5�����ҷֽ����H2O��CO��CO2��������ͼ���ṩ���������Լ������һ��ʵ�飬֤�����ᾧ��ֽ�õ��Ļ��������H2O��CO��CO2 (����װ�ú͵��ܵ���ͼ����ȥ������װ�ÿ��ظ�ʹ�ã���

�ش��������⣺
(6)����װ�ð�����˳��Ϊ________��
(7)����B����ˮ����ͭ������________��
��8����֤��������к���CO��ʵ��������________��
��16�֣���1����ˮ��KI��I2��Һ ��1�֣�
��2�������¶ȹ��ߣ�����Ũ�ȹ�����C6H12O6��H2C2O4��һ�������� ��2�֣�
��3��5H2C2O4��2MnO4����6H����10CO2��2Mn2����8H2O ��3�֣� ��4��x��2 ��2�֣�
��5��Mn2������������� ��6��C��B��F��E��D��A��F��3�֣� ��7����������е�ˮ ��1�֣�
��8��A�к�ɫ��ĩ��죬F�г���ʯ��ˮ����� ��2�֣�
��������
�����������1��������������ɫ�����Լ�������Ƿ�ˮ����ȫ�����õ��Լ�Ϊ��ˮ��KI��I2��Һ��
��2���������μ��ٶȹ��죬�������¶ȹ��ߣ�����Ũ�ȹ��ᵼ��C6H12O6��H2C2O4��һ�����������Ӷ����²�����ʽ��͡�
��3��������л�ԭ�ԣ����������Һ���������ԣ����߷���������ԭ��Ӧ�������ӷ���ʽΪ5H2C2O4��2MnO4����6H����10CO2��2Mn2����8H2O��
��4�����ĸ�����ص����ʵ�����0.005mol������ݷ���ʽ��֪��25.00ml������Һ�в�������ʵ�����0.005mol��2.5��0.0125mol����6.3g���ᾧ������ʵ�����0.0125mol�� ��0.05mol������
��0.05mol������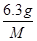 ��0.05mol�����M��126g/mol������x����126��90����18��2��
��0.05mol�����M��126g/mol������x����126��90����18��2��
��5�����ݷ�Ӧ�ķ���ʽ��֪����Ӧ����Mn2�����ɣ����Է�Ӧ���ʼӿ��ԭ�������Mn2������������á�
��6������H2O��CO��CO2���Լ��ֱ�����ˮ����ͭ�����ȵ�����ͭ������ʯ��ˮ��������ȼ���ˮ������CO������������CO2�������ڼ���CO֮ǰ�ȼ���CO2����Ҫ��ȫ��ȥCO2��������ȷ������˳����C��B��F��E��D��A��F��
��7���������Ϸ�����֪������B����ˮ����ͭ�������Ǽ�������е�ˮ��
��8���������Ϸ�����֪����֤��������к���CO��ʵ��������A�к�ɫ��ĩ��죬F�г���ʯ��ˮ����ǡ�
���㣺�������ʼ��飻����ˮ�⣻������ԭ��Ӧ����ʽ����д����������Է�Ӧ���ʵ�Ӱ�죻���ʻ�ѧʽ�����Լ�ʵ�鷽����������۵�

 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�