��Ŀ����
����Ŀ������ˮ�ܿ�(��Ҫ�ɷ�ΪCo2O3��������Fe2O3��Al2O3��MnO��MgO��CaO��)��ȡ�����ܵĹ���������ͼ��ʾ��

����������������������ʽ��ȫ����ʱ��Һ��pH��
�������� | Fe3+ | Fe2+ | Co2+ | Al3+ | Mn2+ |
������ȫ��pH | 2.8 | 8.3 | 9.2 | 5.2 | 9.8 |
��1�����������м���Na2SO3��Ŀ����___��
��2��д������NaClO3������Ӧ�����ӷ���ʽ__�����������Ƿ�Ӧ��ȫ���Լ���__��д�Լ����ƣ���
��3����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ��ʾ��

��Һ���м�����ȡ����������___��ʹ����ȡ�����˵�pH��___(�����)��
A.�ӽ�2.0 B.�ӽ�3.0 C.�ӽ�5.0
��4�������ơ�þ���ǽ���Һ��Ca2+��Mg2+ת��ΪMgF2��CaF2��������֪Ksp(MgF2)=7.35��10-11��Ksp(CaF2)=1.05��10-10�����������NaF��������Һ��![]() =__��
=__��
��5����ҵ���ð�ˮ���շ����е�SO2����֪NH3��H2O�ĵ��볣��Kb=1.8��10-5��H2SO3�ĵ��볣��Ka1=1.2��10-2��Ka2=1.3��10-8����ͨ������Ĺ����У���ǡ���γ�����ʱ����Һ������Ũ�ȵĴ�С��ϵΪ__����ǡ���γ���ʽ��ʱ����������NaOH��Һ����Ӧ�����ӷ���ʽΪ__��
���𰸡���Co3+��Fe3+��ԭ ClO![]() +6Fe2++6H+=6Fe3++Cl-+3H2O ���軯����Һ ��ȥMn2+ B 0.7 c(NH
+6Fe2++6H+=6Fe3++Cl-+3H2O ���軯����Һ ��ȥMn2+ B 0.7 c(NH![]() )>c(SO
)>c(SO![]() )>c(OH-)>c(HSO
)>c(OH-)>c(HSO![]() )>c(H+) HSO
)>c(H+) HSO![]() +OH-=SO
+OH-=SO![]() +H2O
+H2O
��������
���������ͼ��֪�����ܷ����м������ᣬ�ɵ�CoCl3��FeCl3��AlCl3��MnCl2��MgCl2��CaCl2����Ϣ���н���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Ca2+��Mg2+��Al3+�ȣ�������Na2SO3��Co3+��Fe3+��ԭΪCo2+��Fe2+�������������յõ������ܣ�����NaClO3��Fe2+����ΪFe3+������Na2CO3��pH��5.2���ɵõ�Fe��OH��3��Al��OH��3���������˺�������Һ��Ҫ����CoCl2��MnCl2��MgCl2��CaCl2����NaF��Һ��ȥ�ơ�þ�����˺�����Һ�м�����ȡ��������������ȡ��ȥ����ȡ�����Һ����Ҫ����CoCl2������������Һ�õ������ܡ�
(1)�ɷ�����֪�����������м���Na2SO3��Ŀ���ǽ�Co3+��Fe3+��ԭΪCo2+��Fe2+���ʴ�Ϊ����Co3+��Fe3+��ԭ��
(2)����NaClO3��Ŀ���ǽ�Fe2+����ΪFe3+����Ӧ�����ӷ���ʽΪClO![]() +6Fe2++6H+=6Fe3++Cl-+3H2O����Fe2+����ȫ��������Ӧ�����Һ�м������軯����Һ������������ɫ�������ʴ�Ϊ��ClO
+6Fe2++6H+=6Fe3++Cl-+3H2O����Fe2+����ȫ��������Ӧ�����Һ�м������軯����Һ������������ɫ�������ʴ�Ϊ��ClO![]() +6Fe2++6H+=6Fe3++Cl-+3H2O�����軯����Һ��
+6Fe2++6H+=6Fe3++Cl-+3H2O�����軯����Һ��
(3)��Һ�м�����ȡ���������dz�ȥ�����ӣ�pH=2��pH=3ʱCo2+����ȡ�ʱ仯����pH=3ʱMn����ȡ������ܶ࣬��pH=5ʱ��Co2+����ȡ�ʱȽϴ���ʧ���أ���pH=3ʱ��ѣ��ʴ�Ϊ����ȥMn2+��B��
(4)��֪Ksp(MgF2)=7.35��10-11��Ksp(CaF2)=1.05��10-10�����������NaF��������Һ��![]() =
=![]() =
=![]() =0.7���ʴ�Ϊ��0.7��
=0.7���ʴ�Ϊ��0.7��
(5)��NH3H2O�ĵ���ƽ�ⳣ��K=1.8��10-5mol/L��H2SO3�ĵ���ƽ�ⳣ��K2=1.3��10-8molL-1��֪��SO32-��ˮ��̶ȴ���NH4+��ˮ��̶ȣ������������Һ�ʼ��ԣ���Һ������������Դ��ˮ�ĵ��롢SO32-��ˮ����Һ��c��OH-��>c��HSO3-��������Һ������Ũ�ȴ�СΪ��c��NH4+��>c��SO32-��>c��OH-��>c��HSO3-��>c��H+�����ɵ���ƽ�ⳣ����֪��NH4++OH-NH3H2O��ƽ�ⳣ��С��HSO3-+OH-SO32-+H2Oƽ�ⳣ�����������������Һ��������NaOH��Һ������������Һ����HSO3-��Ӧ����Ӧ�����ӷ���ʽΪHSO3-+OH-=SO32-+H2O���ʴ�Ϊ��c��NH4+��>c��SO32-��>c��OH-��>c��HSO3-��>c��H+����HSO3-+OH-=SO32-+H2O��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��������������ᴿ�У����������ܵ���ʵ��ܽ�ƽ��ԭ����ȥijЩ�������ӡ����к��Ȼ��������ʵ��Ȼ�ͭ����(CuCl2��2H2O)��Ϊ��ȡ������CuCl2��2H2O�����Ƚ����Ƴ�ˮ��Һ��Ȼ����ͼ��������ᴿ��

��֪ij�¶��£�Cu2+��Fe3+��Fe2+���������↑ʼ�����ͳ�����ȫʱ��pH�����±���
Fe3+ | Fe2+ | Cu2+ | |
�������↑ʼ����ʱ��pH | 1.9 | 7.0 | 4.7 |
����������ȫ����ʱ��pH | 3.2 | 9.0 | 6.7 |
��ش��������⣺
��1������������X��Ŀ����_______________________��
��2�����ʺ���������X����___________�����ţ������֣���д��������X��������ӷ�Ӧ����ʽ_________��
A��K2Cr2O7 B��NaClO C��H2O2 D��KMnO4
��3��Ϊ�˳�ȥFe3+�����������Y_________���ѧʽ��������Һ��pHֵΪ___________��
��4������ܲ���ֱ�������ᾧ�õ�CuCl2��2H2O���壿________(������������������)�����ܣ����ûش������ܣ��ش����β�����____________________��
��5����������֪Fe(OH)3��Ksp=1.0��10-35����500mL 0.2mol/L��FeCl3��Һ�м���NaOH����(��Һ����仯���Բ���)��pH=3.0������Ҫ�����������ƹ��������Ϊ________g��
����Ŀ������ʵ�������Ӧ��ʵ�������ͻ���۶���ȷ����(����)
ѡ�� | ʵ����� | ʵ������ | ���ͻ���� |
A | ��Fe(NO3)2��Һ�е��������ữ��H2O2��Һ | ��Һ��Ϊ��ɫ | �����ԣ�H2O2>Fe3�� |
B | ��5mL1mol/L NaOH��Һ�еμ�5��1mol/L MgCl2��Һ��Ȼ���ٵμ�������1mol/L CuCl2��Һ | �Ȳ�����ɫ������Ȼ�������ɫ���� | Ksp[Cu(OH)2] >Ksp[Mg(OH)2] |
C | ������NO2���ܱղ������������ˮ�� | ����ɫ���� | 2NO2(g) |
D | ��һ��������KMnO4��Һ�м����Ҷ�����HOCH2CH2OH�� | ��Һ��ɫ��ȥ | �Ҷ���������Ϊ�Ҷ��� |
A. A B. B C. C D. D
����Ŀ��������Ԫ��A��B��D��E��G��J�����ڱ��е�λ�����£�
A | |||||||
B | D | ||||||
E | G | J |
�����ϱ��ش����⣺
(1)![]() �����ڱ��е�λ����______��D��ԭ�ӽṹʾ��ͼ_____��
�����ڱ��е�λ����______��D��ԭ�ӽṹʾ��ͼ_____��
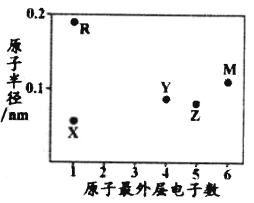
(2)![]() ��B��E��G��ԭ�Ӱ뾶�ɴ�С��˳����_____
��B��E��G��ԭ�Ӱ뾶�ɴ�С��˳����_____![]() ��Ԫ�ط���
��Ԫ�ط���![]() ��
��
(3)![]() ��D����̬�⻯����ȶ��Թ�ϵΪ______
��D����̬�⻯����ȶ��Թ�ϵΪ______![]() �ѧʽ
�ѧʽ![]() �����Ƕ�����______
�����Ƕ�����______![]() ���ӻ�����ۻ�����
���ӻ�����ۻ�����![]() ��
��
(4)��������Ԫ�ص�����������Ӧ��ˮ�����У���һ�����ʼ�����ǿ�ᷴӦ������ǿ�Ӧ��д���������ʸ�����������Һ��Ӧ�Ļ�ѧ����ʽ________________��