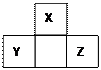��Ŀ����
�٢ڢۢܢ�����Ԫ�أ���Ԫ�����ڱ��е�λ����ͼ��ʾ���ش��������⡣
��1���٢ڢۢܢ�����Ԫ���У���������ǿ��Ԫ���� ����дԪ�����ƣ�����Ԫ�صĵ����ڿ�����ȼ�յĻ�ѧ����ʽΪ ��
��2��Ԫ�آٺ͢ۿ����γ��ڶ�Ļ�������к�������ߵĻ������ǣ�д��ѧʽ��
���ԱȽϢں͢�ԭ�Ӱ뾶�Ĵ�С�� �ܣ��>����<������
��3������Ԫ�آܵ�ԭ�ӽṹʾ��ͼ ����Ԫ�ص�����������ˮ��������Cu������Ӧ����Ӧ�������������ǣ�д��ѧʽ�� ��
��4��д��Ԫ�آ������ڱ��е�λ�� ���䵥�ʿ��ڹ�ҵ��������������Һ��Ư����д���䷴Ӧ�Ļ�ѧ����ʽ ��
��5��Ԫ�آ�����γ����������ĵ���ʽ ���ṹʽ ��
��6��Ԫ�آߵ�ԭ������Ϊ ��λ�� �壻���Ϊ ϵ���� ��Ԫ�ء�
��7���ڱ��л���������ǽ����ֽ��ߣ��ڷֽ��߸�����Ѱ�� ���ϡ�
��1���٢ڢۢܢ�����Ԫ���У���������ǿ��Ԫ���� ����дԪ�����ƣ�����Ԫ�صĵ����ڿ�����ȼ�յĻ�ѧ����ʽΪ ��
��2��Ԫ�آٺ͢ۿ����γ��ڶ�Ļ�������к�������ߵĻ������ǣ�д��ѧʽ��
���ԱȽϢں͢�ԭ�Ӱ뾶�Ĵ�С�� �ܣ��>����<������
��3������Ԫ�آܵ�ԭ�ӽṹʾ��ͼ ����Ԫ�ص�����������ˮ��������Cu������Ӧ����Ӧ�������������ǣ�д��ѧʽ�� ��
��4��д��Ԫ�آ������ڱ��е�λ�� ���䵥�ʿ��ڹ�ҵ��������������Һ��Ư����д���䷴Ӧ�Ļ�ѧ����ʽ ��
��5��Ԫ�آ�����γ����������ĵ���ʽ ���ṹʽ ��
��6��Ԫ�آߵ�ԭ������Ϊ ��λ�� �壻���Ϊ ϵ���� ��Ԫ�ء�
��7���ڱ��л���������ǽ����ֽ��ߣ��ڷֽ��߸�����Ѱ�� ���ϡ�
��1���ƣ� 2Na��O2 Na2O2 ��2��CH4�� >
Na2O2 ��2��CH4�� >
��3�� �� HNO3
�� HNO3
��4��3���ڣ� ��A�壬 Cl2��2NaOH==NaCl��NaClO��H2O
��5�� ��O��C��O ��6��43����B�壻 �ϵ�� 15��
��O��C��O ��6��43����B�壻 �ϵ�� 15��
 Na2O2 ��2��CH4�� >
Na2O2 ��2��CH4�� >��3��
 �� HNO3
�� HNO3��4��3���ڣ� ��A�壬 Cl2��2NaOH==NaCl��NaClO��H2O
��5��
 ��O��C��O ��6��43����B�壻 �ϵ�� 15��
��O��C��O ��6��43����B�壻 �ϵ�� 15����

��ϰ��ϵ�д�
�����Ŀ


 ��SCN
��SCN ��M+�ĵ��Ӳ�ṹ��ͬ����ԭ�������ıȽ�Ϊ��R>M
��M+�ĵ��Ӳ�ṹ��ͬ����ԭ�������ıȽ�Ϊ��R>M