��Ŀ����
�����ʽ��ͬ��A��B����������֪������ͬ�¡�ͬѹ�£���������A��B���壬A��ռ���С��B������һ�������£�A�ܷ��������Ӿۣ�B����ˮ�ӳɣ���14g��A��B�������ϵ����壬�ڱ�״�������Ϊ8.96L����A��ȫȼ�պ����ɶ�����̼��ˮ�����ʵ�����ȣ��Իش�
(1)A��������________��B��������________________________��
(2)A�����Ӿ۷�Ӧ�Ļ�ѧ����ʽ��________________________��
(3)B��ˮ��Ӧ�Ļ�ѧ����ʽ��________________________��
�𰸣�
������
������
|
(1)A����ϩ,B����ϩ (2)nCH2��CH (3)CH2��CH2��H2O |

��ϰ��ϵ�д�
�����Ŀ
 CH3
CH3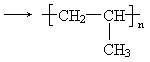
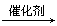 CH3CH2OH
CH3CH2OH