��Ŀ����
����ѧһѡ��3���ʽṹ�����ʡ�
N��BԪ���ڻ�ѧ���к���Ҫ�ĵ�λ��
��1��д����NԪ��ͬ�����AsԪ�صĻ�̬ԭ�Ӻ�������Ų�ʽ ��
��ԭ�ӽṹ�ĽǶȷ���B��N��OԪ�صĵ�һ�������ɴ�С��˳��Ϊ ��
��2��NԪ����BԪ�صķ����ﻯѧʽ���ƣ���ΪAB3�ͣ������ӵĿռ�ṹ�кܴ�ͬ����ԭ���� ������BF3�ķ��ӹ���Ϊ ��
��3������������������˹������ڸ��¸�ѹ�����ºϳɣ���Ӳ�Ƚ����ڽ��ʯ��ԶԶ�����������ϣ����������ʯͳ��Ϊ��Ӳ���ϣ�BN�ľ���ṹ����ʯ���ƣ�����Bԭ�ӵ��ӻ���ʽΪ ��������ڵ��������� ��
��4��NaN3�ǿ�������ҩ���ơ��ϳɹ����е��м�������ʣ�NaN3Ҳ�����������ı������ң�3mol NaN3��ײ��������4mol N2�����һ�����ӻ�����A��
����д������NaN3ײ����Ӧ�Ļ�ѧ����ʽ ��
�ڸ��ݵ����Ƶ��ص���ʽ�жϣ�N2�����д��ڵĦҼ��ͦм���Ŀ֮��Ϊ �� ��
N��BԪ���ڻ�ѧ���к���Ҫ�ĵ�λ��
��1��д����NԪ��ͬ�����AsԪ�صĻ�̬ԭ�Ӻ�������Ų�ʽ
��ԭ�ӽṹ�ĽǶȷ���B��N��OԪ�صĵ�һ�������ɴ�С��˳��Ϊ
��2��NԪ����BԪ�صķ����ﻯѧʽ���ƣ���ΪAB3�ͣ������ӵĿռ�ṹ�кܴ�ͬ����ԭ����
��3������������������˹������ڸ��¸�ѹ�����ºϳɣ���Ӳ�Ƚ����ڽ��ʯ��ԶԶ�����������ϣ����������ʯͳ��Ϊ��Ӳ���ϣ�BN�ľ���ṹ����ʯ���ƣ�����Bԭ�ӵ��ӻ���ʽΪ
��4��NaN3�ǿ�������ҩ���ơ��ϳɹ����е��м�������ʣ�NaN3Ҳ�����������ı������ң�3mol NaN3��ײ��������4mol N2�����һ�����ӻ�����A��
����д������NaN3ײ����Ӧ�Ļ�ѧ����ʽ
�ڸ��ݵ����Ƶ��ص���ʽ�жϣ�N2�����д��ڵĦҼ��ͦм���Ŀ֮��Ϊ
��������1��NԪ�����ڵ�VA��Ԫ�أ���As�ǵ�VA��Ԫ�أ��������s�ܼ���p�ܼ��Ϸֱ���2��3�����ӣ�As��35��Ԫ�أ���ԭ�Ӻ�����35�����ӣ����ݹ���ԭ����д���̬ԭ�Ӻ�������Ų�ʽ��
ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����VA��Ԫ�صĵ�һ�����ܴ���������Ԫ�أ�
��2��NԪ����BԪ�صķ����ﻯѧʽ���ƣ���ΪAB3�ͣ���NԪ�صķ������е�ԭ�Ӻ��йµ��Ӷԣ�BԪ�صķ�������BԪ�ز����µ��Ӷԣ�������ռ乹�Ͳ�ͬ�����ݼ۲���ӶԻ�������ȷ��BF3�ķ��ӿռ乹�ͣ�
��3��BN��Ӳ�Ƚϴ�����BN��ԭ�Ӿ��壬���ݽ��ʯ�Ľṹȷ��BN��Bԭ�ӵ��ӻ���ʽ��ԭ�Ӿ����к��й��ۼ���
��4���ٸ���ԭ���غ�ȷ�����ӻ�����A���ٸ��ݷ�Ӧ���������д��Ӧ����ʽ��
�ڹ��۵���Ϊ�ң����������к���һ���Ҽ���2���м���
ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����VA��Ԫ�صĵ�һ�����ܴ���������Ԫ�أ�
��2��NԪ����BԪ�صķ����ﻯѧʽ���ƣ���ΪAB3�ͣ���NԪ�صķ������е�ԭ�Ӻ��йµ��Ӷԣ�BԪ�صķ�������BԪ�ز����µ��Ӷԣ�������ռ乹�Ͳ�ͬ�����ݼ۲���ӶԻ�������ȷ��BF3�ķ��ӿռ乹�ͣ�
��3��BN��Ӳ�Ƚϴ�����BN��ԭ�Ӿ��壬���ݽ��ʯ�Ľṹȷ��BN��Bԭ�ӵ��ӻ���ʽ��ԭ�Ӿ����к��й��ۼ���
��4���ٸ���ԭ���غ�ȷ�����ӻ�����A���ٸ��ݷ�Ӧ���������д��Ӧ����ʽ��
�ڹ��۵���Ϊ�ң����������к���һ���Ҽ���2���м���
����⣺��1��NԪ�����ڵ�VA��Ԫ�أ���As�ǵ�VA��Ԫ�أ��������s�ܼ���p�ܼ��Ϸֱ���2��3�����ӣ�As��35��Ԫ�أ���ԭ�Ӻ�����35�����ӣ����ݹ���ԭ��֪���̬ԭ�Ӻ�������Ų�ʽΪ��1s22s22p63d104s24p3��[Ar]3d104s24p3��
ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����VA��Ԫ�صĵ�һ�����ܴ���������Ԫ�أ���������Ԫ�صĵ�һ�������ɴ�С��˳��ΪN��O��B��
�ʴ�Ϊ��1s22s22p63d104s24p3��[Ar]3d104s24p3��N��O��B��
��2��NԪ����BԪ�صķ����ﻯѧʽ���ƣ���ΪAB3�ͣ���NԪ�صķ������е�ԭ�Ӻ��йµ��Ӷԣ�BԪ�صķ�������BԪ�ز����µ��Ӷԣ�����Nԭ�Ӻ�Bԭ�Ӳ��õ��ӻ���ʽ��ͬ��������ռ乹�Ͳ�ͬ��BF3��Bԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ�����BF3Ϊƽ�������ι��ͣ�
�ʴ�Ϊ��Nԭ�Ӻ�Bԭ�Ӳ��ò�ͬ���ӻ���ʽ��ƽ�������Σ�
��3��BN��Ӳ�Ƚϴ�����BN��ԭ�Ӿ��壬���ݽ��ʯ�Ľṹ֪BN��Bԭ�ӵ��ӻ���ʽΪsp3��ԭ�Ӿ�����ֻ���й��ۼ����ʴ�Ϊ��sp3�����ۼ���
��4����3mol NaN3��ײ��������4mol N2�����һ�����ӻ�����A������ԭ���غ�ȷ�����ӻ�����A�Ļ�ѧʽΪNa3N�����ݷ�Ӧ�������֪��
�÷�Ӧ����ʽΪ��3 NaN3=4N2+Na3N��
�ʴ�Ϊ��3 NaN3=4N2+Na3N��
�ڹ��۵���Ϊ�ң����������к���һ���Ҽ���2���м����������ӵĽṹʽΪN��N������һ�����������к���1���Ҽ���2���м�����N2�����д��ڵĦҼ��ͦм���Ŀ֮��Ϊ1��2���ʴ�Ϊ��1��2��
ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����VA��Ԫ�صĵ�һ�����ܴ���������Ԫ�أ���������Ԫ�صĵ�һ�������ɴ�С��˳��ΪN��O��B��
�ʴ�Ϊ��1s22s22p63d104s24p3��[Ar]3d104s24p3��N��O��B��
��2��NԪ����BԪ�صķ����ﻯѧʽ���ƣ���ΪAB3�ͣ���NԪ�صķ������е�ԭ�Ӻ��йµ��Ӷԣ�BԪ�صķ�������BԪ�ز����µ��Ӷԣ�����Nԭ�Ӻ�Bԭ�Ӳ��õ��ӻ���ʽ��ͬ��������ռ乹�Ͳ�ͬ��BF3��Bԭ�Ӻ���3���Ҽ��Ҳ����µ��Ӷԣ�����BF3Ϊƽ�������ι��ͣ�
�ʴ�Ϊ��Nԭ�Ӻ�Bԭ�Ӳ��ò�ͬ���ӻ���ʽ��ƽ�������Σ�
��3��BN��Ӳ�Ƚϴ�����BN��ԭ�Ӿ��壬���ݽ��ʯ�Ľṹ֪BN��Bԭ�ӵ��ӻ���ʽΪsp3��ԭ�Ӿ�����ֻ���й��ۼ����ʴ�Ϊ��sp3�����ۼ���
��4����3mol NaN3��ײ��������4mol N2�����һ�����ӻ�����A������ԭ���غ�ȷ�����ӻ�����A�Ļ�ѧʽΪNa3N�����ݷ�Ӧ�������֪��
�÷�Ӧ����ʽΪ��3 NaN3=4N2+Na3N��
�ʴ�Ϊ��3 NaN3=4N2+Na3N��
�ڹ��۵���Ϊ�ң����������к���һ���Ҽ���2���м����������ӵĽṹʽΪN��N������һ�����������к���1���Ҽ���2���м�����N2�����д��ڵĦҼ��ͦм���Ŀ֮��Ϊ1��2���ʴ�Ϊ��1��2��
���������⿼����ۺϣ��漰��ѧ�����жϡ���������Ų�ʽ����д����ѧʽ��ȷ����֪ʶ�㣬������֪ʶǨ�Ƶķ�������BN��Bԭ���ӻ���ʽ���Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
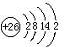
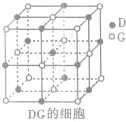 ��3��H3+������һ�����������γɽṹ���ӵ�������[HG��A2C��5]2+�γɸ�������ʱ��H3+���ӽ����������ṩ��
��3��H3+������һ�����������γɽṹ���ӵ�������[HG��A2C��5]2+�γɸ�������ʱ��H3+���ӽ����������ṩ��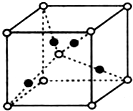 ����ѧһѡ��3���ʽṹ�����ʡ�
����ѧһѡ��3���ʽṹ�����ʡ�
 ���ɣ���
���ɣ��� �д���________(��ѡ����ĸ��
�д���________(��ѡ����ĸ�� �� e. ���
�� e. ���